Comparison of the effectiveness of antibody and cell-mediated immunity against inhaled and instilled influenza virus challenge
- PMID: 23777453
- PMCID: PMC3691648
- DOI: 10.1186/1743-422X-10-198
Comparison of the effectiveness of antibody and cell-mediated immunity against inhaled and instilled influenza virus challenge
Abstract
Background: To evaluate immunity against influenza, mouse challenge studies are typically performed by intranasal instillation of a virus suspension to anesthetized animals. This results in an unnatural environment in the lower respiratory tract during infection, and therefore there is some concern that immune mechanisms identified in this model may not reflect those that protect against infectious virus particles delivered directly to the lower respiratory tract as an aerosol.
Method: To evaluate differences in protection against instilled and inhaled virus, mice were immunized with influenza antigens known to induce antibody or cell-mediated responses and then challenged with 100 LD50 A/PR/8/34 (PR8) in the form of aerosol (inhaled) or liquid suspension (instilled).
Results: Mice immunized with recombinant adenovirus (Ad) expressing hemagglutinin were protected against weight loss and death in both challenge models, however immunization with Ad expressing nucleoprotein of influenza A (NPA) or M2 resulted in greater protection against inhaled aerosolized virus than virus instilled in liquid suspension. Ad-M2, but not Ad-NPA-immunized mice were protected against a lower instillation challenge dose.
Conclusions: These results demonstrate differences in protection that are dependent on challenge method, and suggest that cell-mediated immunity may be more accurately demonstrated in mouse inhalation studies. Furthermore, the data suggest immune mechanisms generally characterized as incomplete or weak in mouse models using liquid intranasal challenge may offer greater immunity against influenza infection than previously thought.
Figures
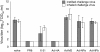



References
-
- Dushoff J, Plotkin J, Viboud C, Earn DJD, Simonsen L. Mortality due to influenza in the United States–an annualized regression approach using multiple-cause mortality data. Am J Epidemiol. 2006;163:181–187. - PubMed
-
- Couch RB, Atmar RL, Franco LM, Quarles JM, Wells J, Arden N, Nino D, Belmont JW. Antibody correlates and predictors of immunity to naturally-occurring influenza in humans and the importance of antibody to the neuraminidase. J Infect Dis. 2013;207:974–981. doi: 10.1093/infdis/jis935. - DOI - PMC - PubMed
-
- Forrest BD, Pride MW, Dunning AJ, Capeding MR, Chotpitayasunondh T, Tam JS, Rappaport R, Eldridge JH, Gruber WC. Correlation of cellular immune responses with protection against culture-confirmed influenza virus in young children. Clin Vaccine Immunol. 2008;15:1042–1053. doi: 10.1128/CVI.00397-07. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

