Dual delivery of BMP-2 and bFGF from a new nano-composite scaffold, loaded with vascular stents for large-size mandibular defect regeneration
- PMID: 23778088
- PMCID: PMC3709809
- DOI: 10.3390/ijms140612714
Dual delivery of BMP-2 and bFGF from a new nano-composite scaffold, loaded with vascular stents for large-size mandibular defect regeneration
Abstract
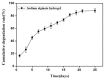
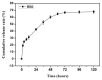
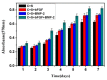
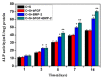
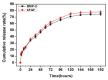
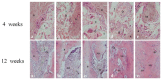
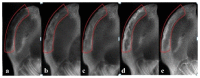
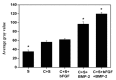
The aim of this study was to investigate the feasibility and advantages of the dual delivery of bone morphogenetic protein-2 (BMP-2) and basic fibroblast growth factor (bFGF) from nano-composite scaffolds (PLGA/PCL/nHA) loaded with vascular stents (PLCL/Col/nHA) for large bone defect regeneration in rabbit mandibles. Thirty-six large bone defects were repaired in rabbits using engineering bone composed of allogeneic bone marrow mesenchymal stem cells (BMSCs), bFGF, BMP-2 and scaffolds composed of PLGA/PCL/nHA loaded with PLCL/Col/nHA. The experiments were divided into six groups: BMSCs/bFGF/BMP-2/scaffold, BMSCs/BMP-2/scaffold, BMSCs/bFGF/scaffold, BMSCs/scaffold, scaffold alone and no treatment. Sodium alginate hydrogel was used as the carrier for BMP-2 and bFGF and its features, including gelling, degradation and controlled release properties, was detected by the determination of gelation and degradation time coupled with a controlled release study of bovine serum albumin (BSA). AlamarBlue assay and alkaline phosphatase (ALP) activity were used to evaluate the proliferation and osteogenic differentiation of BMSCs in different groups. X-ray and histological examinations of the samples were performed after 4 and 12 weeks post-implantation to clarify new bone formation in the mandible defects. The results verified that the use of sodium alginate hydrogel as a controlled release carrier has good sustained release ability, and the combined application of bFGF and BMP-2 could significantly promote the proliferation and osteogenic differentiation of BMSCs (p < 0.05 or p < 0.01). In addition, X-ray and histological examinations of the samples exhibited that the dual release group had significantly higher bone formation than the other groups. The above results indicate that the delivery of both growth factors could enhance new bone formation and vascularization compared with delivery of BMP-2 or bFGF alone, and may supply a promising way of repairing large bone defects in bone tissue engineering.
Figures

Similar articles
-
A Naringin-loaded gelatin-microsphere/nano-hydroxyapatite/silk fibroin composite scaffold promoted healing of critical-size vertebral defects in ovariectomised rat.Int J Biol Macromol. 2021 Dec 15;193(Pt A):510-518. doi: 10.1016/j.ijbiomac.2021.10.036. Epub 2021 Oct 25. Int J Biol Macromol. 2021. PMID: 34710477
-
Preparation and characterization of new nano-composite scaffolds loaded with vascular stents.Int J Mol Sci. 2012;13(3):3366-3381. doi: 10.3390/ijms13033366. Epub 2012 Mar 12. Int J Mol Sci. 2012. PMID: 22489156 Free PMC article.
-
Enhanced healing of rat calvarial defects with MSCs loaded on BMP-2 releasing chitosan/alginate/hydroxyapatite scaffolds.PLoS One. 2014 Aug 1;9(8):e104061. doi: 10.1371/journal.pone.0104061. eCollection 2014. PLoS One. 2014. PMID: 25084008 Free PMC article.
-
Janus sponge/electrospun fibre composite combined with EGF/bFGF/CHX promotes reconstruction in oral tissue regeneration.Colloids Surf B Biointerfaces. 2024 Nov;243:114117. doi: 10.1016/j.colsurfb.2024.114117. Epub 2024 Jul 22. Colloids Surf B Biointerfaces. 2024. PMID: 39084056 Review.
-
Novel Strategies for Spatiotemporal and Controlled BMP-2 Delivery in Bone Tissue Engineering.Cell Transplant. 2024 Jan-Dec;33:9636897241276733. doi: 10.1177/09636897241276733. Cell Transplant. 2024. PMID: 39305020 Free PMC article. Review.
Cited by
-
Mesenchymal stem cell expression of SDF-1β synergizes with BMP-2 to augment cell-mediated healing of critical-sized mouse calvarial defects.J Tissue Eng Regen Med. 2017 Jun;11(6):1806-1819. doi: 10.1002/term.2078. Epub 2015 Jul 31. J Tissue Eng Regen Med. 2017. PMID: 26227988 Free PMC article.
-
Acceleration of callus formation during fracture healing using basic fibroblast growth factor-kidney disease domain-collagen-binding domain fusion protein combined with allogenic demineralized bone powder.J Orthop Surg Res. 2015 May 9;10:59. doi: 10.1186/s13018-015-0201-0. J Orthop Surg Res. 2015. PMID: 25956801 Free PMC article.
-
Synergistic delivery of bFGF and BMP-2 from poly(l-lactic-co-glycolic acid)/graphene oxide/hydroxyapatite nanofibre scaffolds for bone tissue engineering applications.RSC Adv. 2018 Sep 12;8(56):31911-31923. doi: 10.1039/c8ra05250f. eCollection 2018 Sep 12. RSC Adv. 2018. PMID: 35547527 Free PMC article.
-
Natural Polymeric Scaffolds in Bone Regeneration.Front Bioeng Biotechnol. 2020 May 21;8:474. doi: 10.3389/fbioe.2020.00474. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32509754 Free PMC article. Review.
-
Nano-Hydroxyapatite as a Delivery System for Promoting Bone Regeneration In Vivo: A Systematic Review.Nanomaterials (Basel). 2021 Sep 29;11(10):2569. doi: 10.3390/nano11102569. Nanomaterials (Basel). 2021. PMID: 34685010 Free PMC article. Review.
References
-
- Hadlock T.A., Vacanti J.P., Cheney M.L. Tissue engineering in facial plastic and reconstructive surgery. Facial Plast Surg. 1998;14:197–203. - PubMed
-
- Veron C., Chanavaz M., Ferri J., Donazzan M., Hildebrand H.F. A panorama of current materials for osseous application in maxillofacial surgery and oral implantolog (in French) Rev. Stomatol. Chir. Maxillofac. 1995;96:274–281. - PubMed
-
- Hoexter D.L. Bone regeneration graft materials. J. Oral Implantol. 2002;28:290–294. - PubMed
-
- Dinopoulos H., Dimitriou R., Giannoudis P.V. Bone graft substitutes: What are the options? Surgeon. 2012;10:230–239. - PubMed
-
- Dai K.R., Xu X.L., Tang T.T., Zhu Z.A., Yu C.F., Lou J.R., Zhang X.L. Repairing of goat tibial bone defects with BMP-2 gene-modified tissue-engineered bone. Calcif. Tissue Int. 2005;77:55–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

