iPSC-derived β cells model diabetes due to glucokinase deficiency
- PMID: 23778137
- PMCID: PMC3696557
- DOI: 10.1172/JCI67638
iPSC-derived β cells model diabetes due to glucokinase deficiency
Retraction in
-
iPSC-derived β cells model diabetes due to glucokinase deficiency.J Clin Invest. 2017 Mar 1;127(3):1115. doi: 10.1172/JCI92775. Epub 2017 Jan 17. J Clin Invest. 2017. PMID: 28094769 Free PMC article. No abstract available.
Abstract
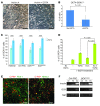
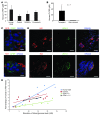
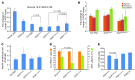
Diabetes is a disorder characterized by loss of β cell mass and/or β cell function, leading to deficiency of insulin relative to metabolic need. To determine whether stem cell-derived β cells recapitulate molecular-physiological phenotypes of a diabetic subject, we generated induced pluripotent stem cells (iPSCs) from subjects with maturity-onset diabetes of the young type 2 (MODY2), which is characterized by heterozygous loss of function of the gene encoding glucokinase (GCK). These stem cells differentiated into β cells with efficiency comparable to that of controls and expressed markers of mature β cells, including urocortin-3 and zinc transporter 8, upon transplantation into mice. While insulin secretion in response to arginine or other secretagogues was identical to that in cells from healthy controls, GCK mutant β cells required higher glucose levels to stimulate insulin secretion. Importantly, this glucose-specific phenotype was fully reverted upon gene sequence correction by homologous recombination. Our results demonstrate that iPSC-derived β cells reflect β cell-autonomous phenotypes of MODY2 subjects, providing a platform for mechanistic analysis of specific genotypes on β cell function.
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

