Dysregulation of voltage-gated sodium channels by ubiquitin ligase NEDD4-2 in neuropathic pain
- PMID: 23778145
- PMCID: PMC3696561
- DOI: 10.1172/JCI68996
Dysregulation of voltage-gated sodium channels by ubiquitin ligase NEDD4-2 in neuropathic pain
Abstract
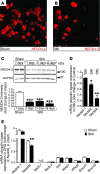
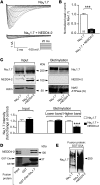
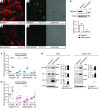
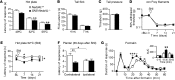
Peripheral neuropathic pain is a disabling condition resulting from nerve injury. It is characterized by the dysregulation of voltage-gated sodium channels (Navs) expressed in dorsal root ganglion (DRG) sensory neurons. The mechanisms underlying the altered expression of Na(v)s remain unknown. This study investigated the role of the E3 ubiquitin ligase NEDD4-2, which is known to ubiquitylate Navs, in the pathogenesis of neuropathic pain in mice. The spared nerve injury (SNI) model of traumatic nerve injury-induced neuropathic pain was used, and an Na(v)1.7-specific inhibitor, ProTxII, allowed the isolation of Na(v)1.7-mediated currents. SNI decreased NEDD4-2 expression in DRG cells and increased the amplitude of Na(v)1.7 and Na(v)1.8 currents. The redistribution of Na(v)1.7 channels toward peripheral axons was also observed. Similar changes were observed in the nociceptive DRG neurons of Nedd4L knockout mice (SNS-Nedd4L(-/-)). SNS-Nedd4L(-/-) mice exhibited thermal hypersensitivity and an enhanced second pain phase after formalin injection. Restoration of NEDD4-2 expression in DRG neurons using recombinant adenoassociated virus (rAAV2/6) not only reduced Na(v)1.7 and Na(v)1.8 current amplitudes, but also alleviated SNI-induced mechanical allodynia. These findings demonstrate that NEDD4-2 is a potent posttranslational regulator of Na(v)s and that downregulation of NEDD4-2 leads to the hyperexcitability of DRG neurons and contributes to the genesis of pathological pain.
Figures

Similar articles
-
Neuronal expression of the ubiquitin ligase Nedd4-2 in rat dorsal root ganglia: modulation in the spared nerve injury model of neuropathic pain.Neuroscience. 2012 Dec 27;227:370-80. doi: 10.1016/j.neuroscience.2012.09.044. Epub 2012 Sep 25. Neuroscience. 2012. PMID: 23022218
-
The Antidiabetic Drug Metformin Regulates Voltage-Gated Sodium Channel NaV1.7 via the Ubiquitin-Ligase NEDD4-2.eNeuro. 2022 Mar 4;9(2):ENEURO.0409-21.2022. doi: 10.1523/ENEURO.0409-21.2022. Print 2022 Mar-Apr. eNeuro. 2022. PMID: 35131865 Free PMC article.
-
Ubiquitylation of voltage-gated sodium channels.Handb Exp Pharmacol. 2014;221:231-50. doi: 10.1007/978-3-642-41588-3_11. Handb Exp Pharmacol. 2014. PMID: 24737239 Review.
-
Voltage-gated sodium channel expression in mouse DRG after SNI leads to re-evaluation of projections of injured fibers.Mol Pain. 2014 Mar 11;10:19. doi: 10.1186/1744-8069-10-19. Mol Pain. 2014. PMID: 24618114 Free PMC article.
-
NEDD4-2 (NEDD4L): the ubiquitin ligase for multiple membrane proteins.Gene. 2015 Feb 15;557(1):1-10. doi: 10.1016/j.gene.2014.11.051. Epub 2014 Nov 26. Gene. 2015. PMID: 25433090 Free PMC article. Review.
Cited by
-
Proteome-scale characterisation of motif-based interactome rewiring by disease mutations.Mol Syst Biol. 2024 Sep;20(9):1025-1048. doi: 10.1038/s44320-024-00055-4. Epub 2024 Jul 15. Mol Syst Biol. 2024. PMID: 39009827 Free PMC article.
-
Differential Inhibition of Nav1.7 and Neuropathic Pain by Hybridoma-Produced and Recombinant Monoclonal Antibodies that Target Nav1.7 : Differential activities of Nav1.7-targeting monoclonal antibodies.Neurosci Bull. 2018 Feb;34(1):22-41. doi: 10.1007/s12264-018-0203-0. Epub 2018 Jan 15. Neurosci Bull. 2018. PMID: 29333591 Free PMC article.
-
Role of the Ubiquitin System in Chronic Pain.Front Mol Neurosci. 2021 May 28;14:674914. doi: 10.3389/fnmol.2021.674914. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34122010 Free PMC article. Review.
-
Downregulation of Cdh1 signalling in spinal dorsal horn contributes to the maintenance of mechanical allodynia after nerve injury in rats.Mol Pain. 2016 May 16;12:1744806916647376. doi: 10.1177/1744806916647376. Print 2016. Mol Pain. 2016. PMID: 27184142 Free PMC article.
-
Gene set enrichment analysis and protein-protein interaction network analysis after sciatic nerve injury.Ann Transl Med. 2020 Aug;8(16):988. doi: 10.21037/atm-20-4958. Ann Transl Med. 2020. PMID: 32953788 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

