A strategy for risk mitigation of antibodies with fast clearance
- PMID: 23778268
- PMCID: PMC3502242
- DOI: 10.4161/mabs.22189
A strategy for risk mitigation of antibodies with fast clearance
Abstract
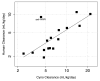
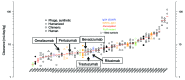
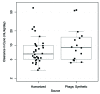
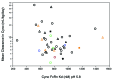
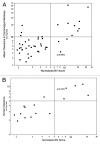
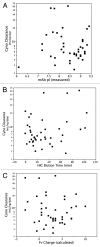
A majority of human therapeutic antibody candidates show pharmacokinetic properties suitable for clinical use, but an unexpectedly fast antibody clearance is sometimes observed that may limit the clinical utility. Pharmacokinetic data in cynomolgus monkeys collected for a panel of 52 antibodies showed broad distribution of target-independent clearance values (2.4-61.3 mL/day/kg), with 15 (29%) having clearance > 10 mL/day/kg. Alteration in the interaction with the recycling FcRn receptor did not account for the faster than expected clearance observed for the antibodies; off-target binding was presumed to account for the fast clearance. We developed an assay based on ELISA detection of non-specific binding to baculovirus particles that can identify antibodies having increased risk for fast clearance. This assay can be used during lead generation or optimization to identify antibodies with increased risk of having fast clearance in both humans and cynomolgus monkeys, and thus increase the likelihood of obtaining a suitable drug candidate.
Keywords: FcRn; antibody; baculovirus; non-specific binding; pharmacokinetics.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
