Immunogenicity and safety of Intanza(®)/IDflu(®) intradermal influenza vaccine in South Korean adults: a multicenter, randomized trial
- PMID: 23778938
- PMCID: PMC3906364
- DOI: 10.4161/hv.25295
Immunogenicity and safety of Intanza(®)/IDflu(®) intradermal influenza vaccine in South Korean adults: a multicenter, randomized trial
Abstract
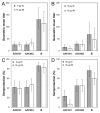
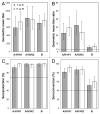
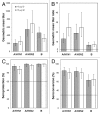
Intanza(®)/IDflu(®) (Sanofi Pasteur, Lyon, France) is an intradermal inactivated trivalent influenza vaccine developed as an alternative to intramuscular influenza vaccine. The objective of this study was to confirm the immunogenicity and safety of Intanza/IDflu in South Korean adults. In a phase IV multicenter trial, South Korean adults 18-59 y old (n = 120) and ≥ 60 y old (n = 120) were randomized 1:1 to receive a single dose of Intanza/IDflu (9 µg for 18-59 y, 15 µg for ≥ 60 y) or trivalent intramuscular vaccine (Vaxigrip(®) 15 µg, Sanofi Pasteur, Lyon, France). Blood was collected on pre-vaccination (day 0) and on day 21. Hemagglutination inhibition titers, seroprotection rates and seroconversion rates were determined on day 21. Geometric mean titers, seroprotection and seroconversion rates were similar between the intradermal and intramuscular vaccines in both age groups for all three vaccine strains (A/H1N1, A/H3N2 and B). Both vaccines met Committee for Medicinal Products for Human Use criteria for all three strains. Solicited systemic reactions of the intradermal groups were generally mild, transient, and similar to those of the intramuscular groups. Solicited injection site reactions were more frequent in the intradermal groups but were mostly mild, transient, and consisted mainly of pain, erythema, and pruritus. No treatment-related serious adverse events or other safety concerns were reported. These results confirm that Intanza/IDflu is an effective and well-tolerated alternative to IM influenza vaccination. (Clinicaltrials.gov NCT ID: NCT01215669).
Keywords: Influenza vaccine; immunogenicity; intradermal; intramuscular; safety.
Figures
Similar articles
-
Safety and immunogenicity of revaccination with reduced dose intradermal and standard dose intramuscular influenza vaccines in adults 18-64 years of age.Vaccine. 2013 Dec 5;31(50):6034-40. doi: 10.1016/j.vaccine.2013.09.012. Epub 2013 Sep 20. Vaccine. 2013. PMID: 24055306 Clinical Trial.
-
Phase 4 randomized trial of intradermal low-antigen-content inactivated influenza vaccine versus standard-dose intramuscular vaccine in HIV-1-infected adults.Hum Vaccin Immunother. 2012 Aug;8(8):1048-52. doi: 10.4161/hv.20347. Epub 2012 Aug 1. Hum Vaccin Immunother. 2012. PMID: 22832261 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of a quadrivalent intradermal influenza vaccine in adults.Vaccine. 2015 Feb 25;33(9):1151-9. doi: 10.1016/j.vaccine.2015.01.025. Epub 2015 Jan 19. Vaccine. 2015. PMID: 25613721 Clinical Trial.
-
Intanza (®) 9 µg intradermal seasonal influenza vaccine for adults 18 to 59 years of age.Hum Vaccin Immunother. 2013 Jan;9(1):115-21. doi: 10.4161/hv.22342. Hum Vaccin Immunother. 2013. PMID: 23442585 Free PMC article. Review.
-
Intanza 15 microg intradermal seasonal influenza vaccine: in older adults (aged >or=60 years).Drugs Aging. 2010 Jul 1;27(7):597-605. doi: 10.2165/11203880-000000000-00000. Drugs Aging. 2010. PMID: 20583853 Review.
Cited by
-
Fractional dose of intradermal compared to intramuscular and subcutaneous vaccination - A systematic review and meta-analysis.Travel Med Infect Dis. 2020 Sep-Oct;37:101868. doi: 10.1016/j.tmaid.2020.101868. Epub 2020 Sep 6. Travel Med Infect Dis. 2020. PMID: 32898704 Free PMC article.
-
Acceptance of intradermal inactivated influenza vaccines among hospital staff following 2 seasonal vaccination campaigns.Hum Vaccin Immunother. 2015;11(12):2827-30. doi: 10.1080/21645515.2015.1072665. Epub 2015 Sep 17. Hum Vaccin Immunother. 2015. PMID: 26378778 Free PMC article.
-
Rapid safety assessment of a seasonal intradermal trivalent influenza vaccine.Hum Vaccin Immunother. 2017 Apr 3;13(4):889-894. doi: 10.1080/21645515.2016.1253644. Epub 2016 Dec 14. Hum Vaccin Immunother. 2017. PMID: 27960593 Free PMC article. Clinical Trial.
-
Immunogenicity and Safety of Reduced-Dose Intradermal vs Intramuscular Influenza Vaccines: A Systematic Review and Meta-analysis.JAMA Netw Open. 2021 Feb 1;4(2):e2035693. doi: 10.1001/jamanetworkopen.2020.35693. JAMA Netw Open. 2021. PMID: 33560425 Free PMC article.
-
Translation of Polymeric Microneedles for Treatment of Human Diseases: Recent Trends, Progress, and Challenges.Pharmaceutics. 2021 Jul 24;13(8):1132. doi: 10.3390/pharmaceutics13081132. Pharmaceutics. 2021. PMID: 34452093 Free PMC article. Review.
References
-
- World Health Organization Vaccines against influenza WHO position paper – November 2012. Wkly Epidemiol Rec. 2012;87:461–76. - PubMed
-
- World Health Organization. World Health Organization 56th World Health Assembly. Prevention and control of influenza pandemics and annual epidemics. Geneva, 2003. WHA56.19.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
