IκB kinase complex (IKK) triggers detachment-induced autophagy in mammary epithelial cells independently of the PI3K-AKT-MTORC1 pathway
- PMID: 23778976
- PMCID: PMC3748193
- DOI: 10.4161/auto.24870
IκB kinase complex (IKK) triggers detachment-induced autophagy in mammary epithelial cells independently of the PI3K-AKT-MTORC1 pathway
Abstract
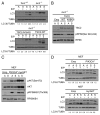
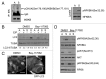
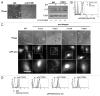
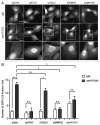
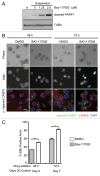
Adherent cells require proper integrin-mediated extracellular matrix (ECM) engagement for growth and survival; normal cells deprived of proper ECM contact undergo anoikis. At the same time, autophagy is induced as a survival pathway in both fibroblasts and epithelial cells upon ECM detachment. Here, we further define the intracellular signals that mediate detachment-induced autophagy and uncover an important role for the IκB kinase (IKK) complex in the induction of autophagy in mammary epithelial cells (MECs) deprived of ECM contact. Whereas the PI3K-AKT-MTORC1 pathway activation potently inhibits autophagy in ECM-detached fibroblasts, enforced activation of this pathway is not sufficient to suppress detachment-induced autophagy in MECs. Instead, inhibition of IKK, as well as its upstream regulator, MAP3K7/TAK1, significantly attenuates detachment-induced autophagy in MECs. Furthermore, function-blocking experiments corroborate that both IKK activation and autophagy induction result from decreased ITGA3-ITGB1 (α3β1 integrin) function. Finally, we demonstrate that pharmacological IKK inhibition enhances anoikis and accelerates luminal apoptosis during acinar morphogenesis in three-dimensional culture. Based on these results, we propose that the IKK complex functions as a key mediator of detachment-induced autophagy and anoikis resistance in epithelial cells.
Keywords: anoikis; autophagy; extracellular matrix; integrin; mammary epithelial cells.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
