para-Aminosalicylic acid is a prodrug targeting dihydrofolate reductase in Mycobacterium tuberculosis
- PMID: 23779105
- PMCID: PMC5395024
- DOI: 10.1074/jbc.M113.475798
para-Aminosalicylic acid is a prodrug targeting dihydrofolate reductase in Mycobacterium tuberculosis
Erratum in
- J Biol Chem. 2013 Oct 4;288(40):28951
Abstract
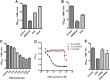
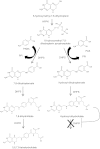
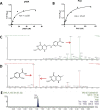
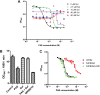
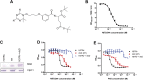
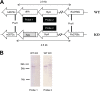
para-Aminosalicylic acid (PAS) is one of the antimycobacterial drugs currently used for multidrug-resistant tuberculosis. Although it has been in clinical use for over 60 years, its mechanism(s) of action remains elusive. Here we report that PAS is a prodrug targeting dihydrofolate reductase (DHFR) through an unusual and novel mechanism of action. We provide evidences that PAS is incorporated into the folate pathway by dihydropteroate synthase (DHPS) and dihydrofolate synthase (DHFS) to generate a hydroxyl dihydrofolate antimetabolite, which in turn inhibits DHFR enzymatic activity. Interestingly, PAS is recognized by DHPS as efficiently as its natural substrate para-amino benzoic acid. Chemical inhibition of DHPS or mutation in DHFS prevents the formation of the antimetabolite, thereby conferring resistance to PAS. In addition, we identified a bifunctional enzyme (riboflavin biosynthesis protein (RibD)), a putative functional analog of DHFR in a knock-out strain. This finding is further supported by the identification of PAS-resistant clinical isolates encoding a RibD overexpression mutation displaying cross-resistance to genuine DHFR inhibitors. Our findings reveal that a metabolite of PAS inhibits DHFR in the folate pathway. RibD was shown to act as a functional analog of DHFR, and as for DHFS, both were shown to be associated in PAS resistance in laboratory strains and clinical isolates.
Keywords: Antibiotic Action; Antibiotic Resistance; Drug Development; Folate Metabolism; Microbiology; Mycobacterium tuberculosis.
Figures

Similar articles
-
Structural Insights into Mycobacterium tuberculosis Rv2671 Protein as a Dihydrofolate Reductase Functional Analogue Contributing to para-Aminosalicylic Acid Resistance.Biochemistry. 2016 Feb 23;55(7):1107-19. doi: 10.1021/acs.biochem.5b00993. Epub 2016 Feb 5. Biochemistry. 2016. PMID: 26848874 Free PMC article.
-
Para-aminosalicylic acid acts as an alternative substrate of folate metabolism in Mycobacterium tuberculosis.Science. 2013 Jan 4;339(6115):88-91. doi: 10.1126/science.1228980. Epub 2012 Nov 1. Science. 2013. PMID: 23118010 Free PMC article.
-
Binding pocket alterations in dihydrofolate synthase confer resistance to para-aminosalicylic acid in clinical isolates of Mycobacterium tuberculosis.Antimicrob Agents Chemother. 2014;58(3):1479-87. doi: 10.1128/AAC.01775-13. Epub 2013 Dec 23. Antimicrob Agents Chemother. 2014. PMID: 24366731 Free PMC article.
-
Inhibitors of de novo folate enzymes in Plasmodium falciparum.Drug Discov Today. 2006 Oct;11(19-20):939-44. doi: 10.1016/j.drudis.2006.08.003. Epub 2006 Sep 7. Drug Discov Today. 2006. PMID: 16997145 Review.
-
Mycobacterium tuberculosis folate metabolism and the mechanistic basis for para-aminosalicylic acid susceptibility and resistance.Antimicrob Agents Chemother. 2015 Sep;59(9):5097-106. doi: 10.1128/AAC.00647-15. Epub 2015 Jun 1. Antimicrob Agents Chemother. 2015. PMID: 26033719 Free PMC article. Review.
Cited by
-
Bioluminescence for assessing drug potency against nonreplicating Mycobacterium tuberculosis.Antimicrob Agents Chemother. 2015 Jul;59(7):4012-9. doi: 10.1128/AAC.00528-15. Epub 2015 Apr 20. Antimicrob Agents Chemother. 2015. PMID: 25896710 Free PMC article.
-
Revitalizing antifolates through understanding mechanisms that govern susceptibility and resistance.Medchemcomm. 2019 May 8;10(6):880-895. doi: 10.1039/c9md00078j. eCollection 2019 Jun 1. Medchemcomm. 2019. PMID: 31303985 Free PMC article. Review.
-
Comparative Genomic Analysis of Two Clonally Related Multidrug Resistant Mycobacterium tuberculosis by Single Molecule Real Time Sequencing.Front Cell Infect Microbiol. 2017 Nov 15;7:478. doi: 10.3389/fcimb.2017.00478. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 29188195 Free PMC article.
-
Antibiotic resistance mechanisms in M. tuberculosis: an update.Arch Toxicol. 2016 Jul;90(7):1585-604. doi: 10.1007/s00204-016-1727-6. Epub 2016 May 9. Arch Toxicol. 2016. PMID: 27161440 Free PMC article. Review.
-
Neuroprotective and Therapeutic Strategies for Manganese-Induced Neurotoxicity.Clin Pharmacol Transl Med. 2017;1(2):54-62. Epub 2017 May 26. Clin Pharmacol Transl Med. 2017. PMID: 30854510 Free PMC article.
References
-
- World Health Organization (2011) WHO Report: Global Tuberculosis Control, pp. 9–26, World Health Organization, Geneva, Switzerland
-
- Dye C., Williams B. G., Espinal M. A., Raviglione M. C. (2002) Erasing the world's slow stain: strategies to beat multidrug-resistant tuberculosis. Science 295, 2042–2046 - PubMed
-
- Lehmann J. (1946) para-Aminosalicylic acid in the treatment of tuberculosis. Lancet 1, 15. - PubMed
-
- Rengarajan J., Sassetti C. M., Naroditskaya V., Sloutsky A., Bloom B. R., Rubin E. J. (2004) The folate pathway is a target for resistance to the drug para-aminosalicylic acid (PAS) in mycobacteria. Mol. Microbiol. 53, 275–282 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

