Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children
- PMID: 23780460
- PMCID: PMC4878912
- DOI: 10.1001/jama.2013.6285
Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children
Abstract
Importance: Type 1 diabetes usually has a preclinical phase identified by circulating islet autoantibodies, but the rate of progression to diabetes after seroconversion to islet autoantibodies is uncertain.
Objective: To determine the rate of progression to diabetes after islet autoantibody seroconversion.
Design, setting, and participants: Data were pooled from prospective cohort studies performed in Colorado (recruitment, 1993-2006), Finland (recruitment, 1994-2009), and Germany (recruitment, 1989-2006) examining children genetically at risk for type 1 diabetes for the development of insulin autoantibodies, glutamic acid decarboxylase 65 (GAD65) autoantibodies, insulinoma antigen 2 (IA2) autoantibodies, and diabetes. Participants were all children recruited and followed up in the 3 studies (Colorado, 1962; Finland, 8597; Germany, 2818). Follow-up assessment in each study was concluded by July 2012.
Main outcomes and measures: The primary analysis was the diagnosis of type 1 diabetes in children with 2 or more autoantibodies. The secondary analysis was the diagnosis of type 1 diabetes in children with 1 autoantibody or no autoantibodies.
Results: Progression to type 1 diabetes at 10-year follow-up after islet autoantibody seroconversion in 585 children with multiple islet autoantibodies was 69.7% (95% CI, 65.1%-74.3%), and in 474 children with a single islet autoantibody was 14.5% (95% CI, 10.3%-18.7%). Risk of diabetes in children who had no islet autoantibodies was 0.4% (95% CI, 0.2%-0.6%) by the age of 15 years. Progression to type 1 diabetes in the children with multiple islet autoantibodies was faster for children who had islet autoantibody seroconversion younger than age 3 years (hazard ratio [HR], 1.65 [95% CI, 1.30-2.09; P < .001]; 10-year risk, 74.9% [95% CI, 69.7%-80.1%]) vs children 3 years or older (60.9% [95% CI, 51.5%-70.3%]); for children with the human leukocyte antigen (HLA) genotype DR3/DR4-DQ8 (HR, 1.35 [95% CI, 1.09-1.68; P = .007]; 10-year risk, 76.6% [95% CI, 69.2%-84%]) vs other HLA genotypes (66.2% [95% CI, 60.2%-72.2%]); and for girls (HR, 1.28 [95% CI, 1.04-1.58; P = .02];10-year risk, 74.8% [95% CI, 68.0%-81.6%]) vs boys (65.7% [95% CI, 59.3%-72.1%]).
Conclusions and relevance: The majority of children at risk of type 1 diabetes who had multiple islet autoantibody seroconversion progressed to diabetes over the next 15 years. Future prevention studies should focus on this high-risk population.
Figures

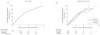

Comment in
-
The evolution of type 1 diabetes.JAMA. 2013 Jun 19;309(23):2491-2. doi: 10.1001/jama.2013.6286. JAMA. 2013. PMID: 23780463 No abstract available.
References
-
- Eisenbarth GS. Type I diabetes mellitus: a chronic autoimmune disease. N Engl J Med. 1986;314(21):1360–1368. - PubMed
-
- Milton B, Holland P, Whitehead M. The social and economic consequences of childhood-onset type 1 diabetes mellitus across the lifecourse: a systematic review. Diabet Med. 2006;23(8):821–829. - PubMed
-
- Tarn AC, Thomas JM, Dean BM, et al. Predicting insulin-dependent diabetes. Lancet. 1988;1(8590):845–850. - PubMed
-
- Gale EA, Bingley PJ, Emmett CL, Collier T. European Nicotinamide Diabetes Intervention Trial (ENDIT) Group. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet. 2004;363(9413):925–931. - PubMed
-
- Diabetes Prevention Trial—Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346(22):1685–1691. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

