Tricyclic drugs for depression in children and adolescents
- PMID: 23780719
- PMCID: PMC7093893
- DOI: 10.1002/14651858.CD002317.pub2
Tricyclic drugs for depression in children and adolescents
Abstract
Background: There is a need to identify effective and safe treatments for depression in children and adolescents. While tricyclic drugs are effective in treating depression in adults, individual studies involving children and adolescents have been equivocal. Prescribing of tricyclic drugs for depression in children and adolescents is now uncommon, but an accurate estimate of their efficacy is helpful as a comparator for other drug treatments for depression in this age group. This is an update of a Cochrane review first published in 2000 and updated in 2002, 2006 and 2010.
Objectives: To assess the effects of tricyclic drugs compared with placebo for depression in children and adolescents and to determine whether there are differential responses to tricyclic drugs between child and adolescent patient populations.
Search methods: We conducted a search of the Cochrane Depression, Anxiety and Neurosis Review Group's Specialised Register (CCDANCTR) (to 12 April 2013), which includes relevant randomised controlled trials from the following bibliographic databases: the Cochrane Central Register of Controlled Trials (CENTRAL) (all years), EMBASE (1974-), MEDLINE (1950-) and PsycINFO (1967-). The bibliographies of previously published reviews and papers describing original research were cross-checked. We contacted authors of relevant abstracts in conference proceedings of the American Academy of Child and Adolescent Psychiatry, and we handsearched the Journal of the American Academy of Child and Adolescent Psychiatry (1978 to 1999).
Selection criteria: Randomised controlled trials comparing the efficacy of orally administered tricyclic drugs with placebo in depressed people aged 6 to 18 years.
Data collection and analysis: One of two review authors selected the trials, assessed their quality, and extracted trial and outcome data. A second review author assessed quality and checked accuracy of extracted data. Most studies reported multiple outcome measures including depression scales and clinical global impression scales. For each study, we took the best available depression measure as the index measure of depression outcome. We established predetermined criteria to assist in the ranking of measures. Where study authors reported categorical outcomes, we calculated individual and pooled risk ratios for non-improvement in treated compared with control subjects. For continuous outcomes, we calculated pooled effect sizes as the number of standard deviations by which the change in depression scores for the treatment group exceeded those for the control group.
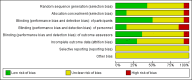
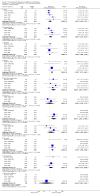
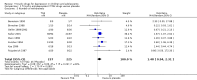
Main results: Fourteen trials (590 participants) were included. No overall difference was found for the primary outcome of response to treatment compared with placebo (risk ratio (RR) 1.07, 95% confidence interval (CI) 0.91 to 1.26; 9 trials, N = 454). There was a small reduction in depression symptoms (standardised mean difference (SMD) -0.32, 95% CI -0.59 to -0.04; 13 trials, N = 533), but the evidence was of low quality. Subgroup analyses suggested a small reduction in depression symptoms among adolescents (SMD -0.45, 95% CI -0.83 to -0.007), and negligible change among children (SMD 0.15, 95% CI -0.34 to 0.64). Treatment with a tricyclic antidepressant caused more vertigo (RR 2.76, 95% CI 1.73 to 4.43; 5 trials, N = 324), orthostatic hypotension (RR 4.86, 95% CI 1.69 to 13.97; 5 trials, N = 324), tremor (RR 5.43, 95% CI 1.64 to 17.98; 4 trials, N = 308) and dry mouth (RR 3.35, 95% CI 1.98 to 5.64; 5 trials, N = 324) than did placebo, but no differences were found for other possible adverse effects. Wide CIs and the probability of selective reporting mean that there was very low-quality evidence for adverse events.There was heterogeneity across the studies in the age of participants, treatment setting, tricyclic drug administered and outcome measures. Statistical heterogeneity was identified for reduction in depressive symptoms, but not for rates of remission or response. As such, the findings from analyses of pooled data should be interpreted with caution.We judged none of these trials to be at low risk of bias, with limited information about many aspects of risk of bias, high dropout rates, and issues regarding measurement instruments and the clinical usefulness of outcomes, which were often variously defined across trials.
Authors' conclusions: Data suggest tricyclic drugs are not useful in treating depression in children. There is marginal evidence to support the use of tricyclic drugs in the treatment of depression in adolescents.
Conflict of interest statement
There were no conflicts of interest.
Figures








Update of
-
Tricyclic drugs for depression in children and adolescents.Cochrane Database Syst Rev. 2002;(2):CD002317. doi: 10.1002/14651858.CD002317. Cochrane Database Syst Rev. 2002. Update in: Cochrane Database Syst Rev. 2013 Jun 18;(6):CD002317. doi: 10.1002/14651858.CD002317.pub2. PMID: 12076448 Updated.
References
References to studies included in this review
Bernstein 1990 {published data only}
-
- Bernstein GA, Garfinkel BD, Borchardt CM. Comparative studies of pharmacotherapy for school refusal. Journal of the American Academy of Child and Adolescent Psychiatry 1990;29:773‐81. - PubMed
Bernstein 2000 {published data only}
-
- Bernstein GA, Anderson LK, Hektner JM, Realmuto GM. Imipramine compliance in adolescents. Journal of the American Academy of Child and Adolescent Psychiatry 2000;39(3):284‐91. - PubMed
-
- Bernstein GA, Borchardt CM, Perwien AR, Crosby RD, Kushner MG, Thuras PD, et al. Imipramine plus cognitive‐behavioural therapy in the treatment of school refusal. Journal of the American Academy of Child and Adolescent Psychiatry 2000;39(3):276‐83. - PubMed
-
- Bernstein GA, Hektner JM, Borchardt CM, McMillan MH. Treatment of school refusal: one‐year follow‐up. Journal of the American Academy of Child & Adolescent Psychiatry 2001;40(2):206‐13. - PubMed
-
- Bernstein GA, Warren SL, Massie ED, Thuras PD. Family dimensions in anxious‐depressed school refusers. Journal of Anxiety Disorders 1999;13(5):513‐28. - PubMed
-
- Layne AE, Bernstein GA, Egan EA, Kushner MG. Predictors of treatment response in anxious‐depressed adolescents with school refusal. Journal of the American Academy of Child & Adolescent Psychiatry 2003;42(3):319‐26. - PubMed
Birmaher 1998 {published data only}
-
- Birmaher B, Waterman GS, Ryan ND, Perel J, McNabb J, Balach L, et al. Randomized, controlled trial of amitriptyline versus placebo for adolescents with "treatment‐resistant" major depression. Journal of the American Academy of Child and Adolescent Psychiatry 1998;37:527‐35. - PubMed
Geller 1989/1992 {published data only}
-
- Geller B, Cooper TB, Graham DL, Fetner HH, Marsteller FA, Wells JM. Pharmacokinetically designed double‐blind placebo‐controlled study of nortriptyline in 6‐12 year‐olds with major depressive disorder. Journal of the American Academy of Child and Adolescent Psychiatry 1992;31:34‐44. - PubMed
-
- Geller B, Cooper TB, McCombs HG, Graham D, Wells J. Double‐blind, placebo‐controlled study of nortriptyline in depressed children using a "fixed plasma level" design. Psychopharmacology Bulletin 1989;25:101‐8. - PubMed
Geller 1990 {published data only}
-
- Geller B, Cooper TB, Graham DL, Marsteller FA, Bryant DM. Double‐blind placebo‐controlled study of nortriptyline in depressed adolescents using a "fixed plasma level" design. Psychopharmacology Bulletin 1990;26:85‐90. - PubMed
Hughes 1990 {published data only}
-
- Hughes CW, Preskorn SH, Weller E, Weller R, Hassanein R. Imipramine vs. placebo studies of childhood depression: baseline predictors of response to treatment and factor analysis of presenting symptoms. Psychopharmacology Bulletin 1998;24(2):275‐9. - PubMed
-
- Hughes CW, Preskorn SH, Weller E, Weller RA, Hassanein R, Tucker S. The effect of concomitant disorders in childhood depression on predicting treatment response. Psychopharmacology Bulletin 1990;26:235‐8. - PubMed
Kashani 1984 {published data only}
-
- Kashani JH, Shekim WO, Reid JC. Amitriptyline in children with major depressive disorder: a double‐blind crossover pilot study. Journal of the American Academy of Child and Adolescent Psychiatry 1984;23:348‐51. - PubMed
Keller 2001 {published data only}
-
- Keller MB, Ryan ND, Birmaher B, Klein RG, Strober M, Wagner KD, et al. Paroxetine and imipramine in the treatment of adolescent depression. Proceedings of the 151st Annual Meeting of the American Psychiatric Association (APA); 1998 May 30‐Jun 4; Toronto, ON, Canada. 1998:123.
-
- Keller MB, Ryan ND, Strober M, Klein RG, Kutcher SP, Birmaher B, et al. Efficacy of paroxetine treatment in the treatment of adolescent depression: a randomized, controlled trial. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(7):762‐72. - PubMed
-
- Wagner KD, Birmaher B, Carlson G, Clarke G, Emslie G, Geller B, et al. Safety of paroxetine and imipramine in the treatment of adolescent depression. Annual Meeting of New Clinical Drug Evaluation Program (NCDEU); 1998 Jun 11; Boca Raton (FL). 1998:Abstract No. 69.
Klein 1998 {published data only}
-
- Klein RG, Mannuzza S, Koplewicz HS, Tancer NK, Shah M, Liang V, et al. Adolescent depression: controlled desipramine treatment and atypical features. Depression & Anxiety 1998;7(1):15‐31. - PubMed
Kramer 1981 {published data only}
-
- Kramer AD, Feguine RJ. Clinical effects of amitriptyline in adolescent depression. Journal of the American Academy of Child and Adolescent Psychiatry 1981;20:636‐44. - PubMed
Kutcher 1994 {published data only}
-
- Boulos C, Kutcher S, Marton P, Simeon J, Ferguson B, Roberts N. Response to desipramine treatment in adolescent major depression. Psychopharmacology Bulletin 1991;27:59‐65. - PubMed
-
- Kutcher S, Boulos C, Ward B, Marton P, Simeon J, Ferguson HB, et al. Response to desipramine treatment in adolescent depression: a fixed‐dose, placebo‐controlled trial. Journal of the American Academy of Child & Adolescent Psychiatry 1994;33(5):686‐94. - PubMed
Kye 1996 {published data only}
-
- Klein RG. Pharmacotherapy of adolescent depression. Pediatric Psychopharmacology 1992;15 Suppl 1, Pt A:227A. - PubMed
-
- Kye CH, Waterman GS, Ryan ND, Birmaher B, Williamson DE, Iyengar S, et al. A randomized, controlled trial of amitriptyline in the acute treatment of adolescent major depression. Journal of the American Academy of Child and Adolescent Psychiatry 1996;35:1139‐44. - PubMed
-
- Ryan ND, Puig‐Antich J. Affective illness in adolescence. In: Frances AJ, Hales RE editor(s). American Psychiatric Association Annual Review. Vol. 5, Washington, DC: American Psychiatric Press, 1986:420‐50.
Petti 1982 {published data only}
-
- Petti TA, Law W. Imipramine treatment of depressed children: a double‐blind pilot study. Journal of Clinical Psychopharmacology 1982;2:107‐10. - PubMed
Puig‐Antich 1987 {published data only}
-
- Puig‐Antich J, Perel JM, Lupatkin W, Chambers WJ, Tabrizi MA, King J, et al. Imipramine in prepubertal major depressive disorders. Archives of General Psychiatry 1987;44:81‐9. - PubMed
References to studies excluded from this review
Berney 1981 {published data only}
-
- Berney T, Kolvin I, Bhate SR, Garside RF, Jeans J, Kay B, et al. School phobia: a therapeutic trial with clomipramine and short‐term outcome. British Journal of Psychiatry 1981;138:110‐8. - PubMed
Lucas 1965 {published data only}
-
- Lucas AR, Lockett HJ, Grimm F. Amitriptyline in childhood depressions. Diseases of the Nervous System 1965;26(2):105‐10. - PubMed
Preskorn 1982 {published data only}
-
- Preskorn SH, Weller EB, Weller RA. Depression in children: relationship between plasma imipramine levels and response. Journal of Clinical Psychiatry 1982;43:450‐3. - PubMed
Sallee 1997 {published data only}
-
- Sallee FR, Vrindavanam NS, Deas‐Smith D, Carson, SW, Sethuraman G. Pulse intravenous clomipramine for depressed adolescents: double‐blind, controlled trial. American Journal of Psychiatry 1997;154:668‐73. - PubMed
References to studies awaiting assessment
Berard 1998 {published data only}
-
- Berard 1998. Clinical trials and tribulations in the pharmacological treatment of adolescent depression. Proceedings of the XXIst Collegium Internationale Neuro Psychopharmacologicum; 1998 Jul 12‐16; Glasgow, Scotland. 1998.
Additional references
APA 2000
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM‐IV‐TR). Washington, DC: American Psychiatric Association, 2000.
Barbey 1998
-
- Barbey JT, Roose SP. SSRI safety in overdose. Journal of Clinical Psychiatry 1998;59 Suppl:42‐8. - PubMed
Birmaher 1996
-
- Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J, Dahl RE, et al. Childhood and adolescent depression: a review of the past 10 years. Part I. Journal of the American Academy of Child and Adolescent Psychiatry 1996;35:1427‐39. - PubMed
Byrne 1989
-
- Byrne MM. Meta‐analysis of early phase II studies with paroxetine in hospitalised depressed patients. Acta Psychiatrica Scandinavica, Supplementum 1989;350:138‐9. - PubMed
Costello 2006
-
- Costello EJ, Foley DL, Angold A. 10‐year research update review; the epidemiology of child and adolescent psychiatric disorders: ll. Developmental epidemiology. Journal of American Academy of Child and Adolescent Psychiatry 2006;45(1):8‐25. - PubMed
Emslie 1997
-
- Emslie GJ, Rush R, Weinberg WA, Kowatch WA, Hughes CW, Carmody T, et al. A double‐blind, randomized, placebo‐controlled trial of fluoxetine in children and adolescents with depression. Archives of General Psychiatry 1997;54:1031‐7. - PubMed
Feighner 1999
-
- Feighner JP. Mechanism of action of antidepressant medications. Journal of Clinical Psychiatry 1999;60(4):4‐11. - PubMed
Hayes 2010
-
- Hayes BD, Klein‐Schwartz W, Clark RF, Muller AA, Miloradovich JE. Comparison of toxicity of acute overdoses with citalopram and escitalopram. Journal of Emergency Medicine 2010;39(1):44‐8. - PubMed
Hetrick 2012
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jensen 1992
-
- Jensen PS, Ryan ND, Prien R. Psychopharmacology of child and adolescent major depression: present status and future directions. Journal of Child and Adolescent Psychopharmacology 1992;2:31‐45. - PubMed
Klein‐Schwartz 1996
-
- Klein‐Schwartz W, Anderson B. Analysis of sertraline‐only overdoses. American Journal of Emergency Medicine 1996;14(5):456‐8. - PubMed
McFee 2001
-
- McFee RB, Caraccio TR, Mofenson HC. Selected tricyclic antidepressant ingestions involving children 6 years old or less. Academic Emergency Medicine 2001;8(2):139‐44. - PubMed
Olgun 2009
-
- Olgun H, Yildirim ZK, Karacan M, Ceviz N. Clinical, electrocardiographic, and laboratory findings in children with amitriptyline intoxication. Pediatric Emergency Care 2009;25(3):170‐3. - PubMed
Petti 1985
-
- Petti T. Scales of potential use in the psychopharmacological treatment of depressed children and adolescents. Psychopharmacology Bulletin 1985;21:951‐77. - PubMed
Phillips 1997
-
- Phillips S, Brent J, Kulig K, Heiligenstein J, Birkett M. Fluoxetine versus tricyclic antidepressants: a prospective multicentre study of antidepressant drug overdoses. The Antidepressant Study Group. Journal of Emergency Medicine 1997;15(4):439‐45. - PubMed
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rosenberg 1989
-
- Rosenberg ML, Eddy DM, Wolpert RC, Broumas EP. Developing strategies to prevent youth suicide. In: Pfeffer CR editor(s). Suicide Among Youth: Perspectives on Risk and Prevention. Washington, DC: American Psychiatric Press, 1989:203‐25.
Ryan 1992
-
- Ryan ND. The pharmacologic treatment of child and adolescent depression. Psychiatric Clinics of North America 1992;15:29‐39. - PubMed
Stahl 2006
-
- Stahl SM. Essential Psychopharmacology. New York: Cambridge University Press, 2006.
Varley 1997
-
- Varley CK, McClellan J. Case study: two additional sudden deaths with tricyclic antidepressants. Journal of the American Academy of Child & Adolescent Psychiatry 1997;36(3):390‐4. - PubMed
Werry 1999
-
- Werry JS, Aman MG. Practitioner's Guide to Psychoactive Drugs for Children and Adolescents. New York: Springer, 1999.
WHO 1992
-
- World Health Organization (WHO). The ICD‐10 Classification of Mental and Behavioural Disorders. Geneva: WHO, 1992.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

