Building a fission machine--structural insights into dynamin assembly and activation
- PMID: 23781021
- PMCID: PMC4067269
- DOI: 10.1242/jcs.108845
Building a fission machine--structural insights into dynamin assembly and activation
Abstract
Dynamin is a large multidomain GTPase that assembles into helical arrays around the necks of deeply invaginated clathrin-coated pits and catalyzes membrane fission during the final stages of endocytosis. Although it is well established that the function of dynamin in vivo depends on its oligomerization and its capacity for efficient GTP hydrolysis, the molecular mechanisms governing these activities have remained poorly defined. In recent years, there has been an explosion of structural data that has provided new insights into the architecture, organization and nucleotide-dependent conformational changes of the dynamin fission machine. Here, we review the key findings of these efforts and discuss the implications of each with regard to GTP hydrolysis, dynamin assembly and membrane fission.
Keywords: Dynamin; Endocytosis; GTPase; Hydrolysis; Membrane fission; Structure.
Figures



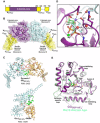
 dimer (PDB: 2X2E). The monomer cores are colored purple and cyan, and the bundle signaling elements (BSEs) are in yellow. (C) Switch region I (red) and switch region II (green) are stabilized at the dimer interface in the GG transition-state complex. GG monomers are shown in cyan and orange. The structure of the Giα1–RGS4 transition-state complex is shown below for comparison (Giα1 in cyan, RGS4 GAP in orange). Note that in this case the switch regions are stabilized at the interface between the G-protein and the GAP. (D) Transition-state stabilization of the of the GG active site. Structural elements involved in this stabilization are labeled. Catalytic and bridging waters are depicted as red spheres; bound Mg2+ and charge-compensating Na+ ions are shown as green and blue spheres, respectively. Dashed lines indicate hydrogen-bonding interactions. Together, the charge compensating cation, the bound Mg2+ and the K44 side-chain act to neutralize the negative charge that develops when the bond is broken between the β- and γ-phosphates during hydrolysis. (E) Conformational changes in the active site that accompany the formation of a G domain dimer in dynamin.
dimer (PDB: 2X2E). The monomer cores are colored purple and cyan, and the bundle signaling elements (BSEs) are in yellow. (C) Switch region I (red) and switch region II (green) are stabilized at the dimer interface in the GG transition-state complex. GG monomers are shown in cyan and orange. The structure of the Giα1–RGS4 transition-state complex is shown below for comparison (Giα1 in cyan, RGS4 GAP in orange). Note that in this case the switch regions are stabilized at the interface between the G-protein and the GAP. (D) Transition-state stabilization of the of the GG active site. Structural elements involved in this stabilization are labeled. Catalytic and bridging waters are depicted as red spheres; bound Mg2+ and charge-compensating Na+ ions are shown as green and blue spheres, respectively. Dashed lines indicate hydrogen-bonding interactions. Together, the charge compensating cation, the bound Mg2+ and the K44 side-chain act to neutralize the negative charge that develops when the bond is broken between the β- and γ-phosphates during hydrolysis. (E) Conformational changes in the active site that accompany the formation of a G domain dimer in dynamin.  monomer colored purple; nucleotide free rat dynamin G domain structure (PDB: 2AKA) colored green. These structural changes serve to optimally position the catalytic machinery, which in turn promotes stimulated GTP hydrolysis.
monomer colored purple; nucleotide free rat dynamin G domain structure (PDB: 2AKA) colored green. These structural changes serve to optimally position the catalytic machinery, which in turn promotes stimulated GTP hydrolysis.

 (cyan, PDB: 2X2E) highlighting alternative BSE conformations. The BSE hinge is colored blue. (B) Side view of the hydrolysis-dependent BSE conformational change that constitutes the dynamin powerstroke. GGGMPPCP and
(cyan, PDB: 2X2E) highlighting alternative BSE conformations. The BSE hinge is colored blue. (B) Side view of the hydrolysis-dependent BSE conformational change that constitutes the dynamin powerstroke. GGGMPPCP and  monomers are colored as in A and the BSE helices are labeled. The red arrow labeled ‘powerstroke’ depicts the 69° downwards rotation of the BSE in the transition-state complex. (C,D) Conformational coupling of the β-sheet, switch I and BSE movements during GTP loading (C) and GTP hydrolysis (D). GGGMPPCP (purple, PDB: 3ZYC),
monomers are colored as in A and the BSE helices are labeled. The red arrow labeled ‘powerstroke’ depicts the 69° downwards rotation of the BSE in the transition-state complex. (C,D) Conformational coupling of the β-sheet, switch I and BSE movements during GTP loading (C) and GTP hydrolysis (D). GGGMPPCP (purple, PDB: 3ZYC),  (cyan, PDB: 2X2E) and the G domain from the nucleotide-free, full-length rat dynamin crystal structure (yellow, PDB: 3ZVR) are superimposed. Arrows indicate the conformational changes between the different structures.
(cyan, PDB: 2X2E) and the G domain from the nucleotide-free, full-length rat dynamin crystal structure (yellow, PDB: 3ZVR) are superimposed. Arrows indicate the conformational changes between the different structures.References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

