Biphasic modulation of NOS expression, protein and nitrite products by hydroxocobalamin underlies its protective effect in endotoxemic shock: downstream regulation of COX-2, IL-1β, TNF-α, IL-6, and HMGB1 expression
- PMID: 23781123
- PMCID: PMC3679756
- DOI: 10.1155/2013/741804
Biphasic modulation of NOS expression, protein and nitrite products by hydroxocobalamin underlies its protective effect in endotoxemic shock: downstream regulation of COX-2, IL-1β, TNF-α, IL-6, and HMGB1 expression
Abstract
Background: NOS/•NO inhibitors are potential therapeutics for sepsis, yet they increase clinical mortality. However, there has been no in vivo investigation of the (in vitro) •NO scavenger, cobalamin's (Cbl) endogenous effects on NOS/•NO/inflammatory mediators during the immune response to sepsis.
Methods: We used quantitative polymerase chain reaction (qPCR), ELISA, Western blot, and NOS Griess assays, in a C57BL/6 mouse, acute endotoxaemia model.
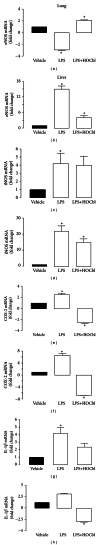
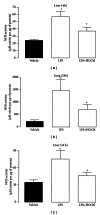
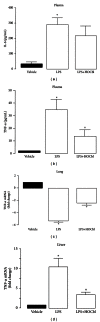
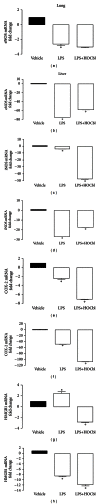
Results: During the immune response, pro-inflammatory phase, parenteral hydroxocobalamin (HOCbl) treatment partially inhibits hepatic, but not lung, iNOS mRNA and promotes lung eNOS mRNA, but attenuates the LPS hepatic rise in eNOS mRNA, whilst paradoxically promoting high iNOS/eNOS protein translation, but relatively moderate •NO production. HOCbl/NOS/•NO regulation is reciprocally associated with lower 4 h expression of TNF-α, IL-1β, COX-2, and lower circulating TNF-α, but not IL-6. In resolution, 24 h after LPS, HOCbl completely abrogates a major late mediator of sepsis mortality, high mobility group box 1 (HMGB1) mRNA, inhibits iNOS mRNA, and attenuates LPS-induced hepatic inhibition of eNOS mRNA, whilst showing increased, but still moderate, NOS activity, relative to LPS only. experiments (LPS+D-Galactosamine) HOCbl afforded significant, dose-dependent protection in mice.
Conclusions: HOCbl produces a complex, time- and organ-dependent, selective regulation of NOS/•NO during endotoxaemia, corollary regulation of downstream inflammatory mediators, and increased survival. This merits clinical evaluation.
Figures






References
-
- Banerjee R, Chowdhury S. Methylmalonyl-CoA Mutase. In: Banerjee R, editor. Chemistry and Biochemistry of B12. New York, NY, USA: John Wiley & Sons; 1999. pp. 707–730.
-
- Matthews R. Cobalamin-dependent methionine synthase. In: Banerjee R, editor. Chemistry and Biochemistry of B12. New York, NY, USA: John Wiley & Sons; 1999. pp. 681–706.
-
- Wheatley C. Cobalamin and Cancer. Oxford, UK: in preparation for: In Tech; 2013. Effects of cobalamin on immunity/inflammation.
-
- Traina V. Vitamin B12 as an anti-anaphylactic. Nature. 1950;166(4210):78–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

