Detecting circulating tumor cells: current challenges and new trends
- PMID: 23781285
- PMCID: PMC3677409
- DOI: 10.7150/thno.5195
Detecting circulating tumor cells: current challenges and new trends
Abstract
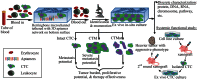
Circulating tumor cells (CTCs) in the blood stream play a critical role in establishing metastases. The clinical value of CTCs as a biomarker for early cancer detection, diagnosis, prognosis, prediction, stratification, and pharmacodynamics have been widely explored in recent years. However, the clinical utility of current CTC tests is limited mainly due to methodological constraints. In this review, the pros and cons of the reported CTC assays are comprehensively discussed. In addition, the potential of tumor cell-derived materials as new targets for CTC detection, including circulating tumor microemboli, cell fragments, and circulating DNA, is evaluated. Finally, emerging approaches for CTC detection, including telomerase-based or aptamer-based assays and cell functional analysis, are also assessed. Expectantly, a thorough review of the current knowledge and technology of CTC detection will assist the scientific community in the development of more efficient CTC assay systems.
Keywords: CTC: Circulating tumor cell; CTDNA: Circulating tumor DNA; CTM: Circulating tumor microemboli; CTMat: Circulating tumor materials; POCT: Point-of-care test..
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. - PubMed
-
- Wicha MS, Hayes DF. Circulating tumor cells: not all detected cells are bad and not all bad cells are detected. J Clin Oncol. 2011;29(12):1508–1511. - PubMed
-
- Rao GC, Larson C, Repollet M, Rutner H, Terstappen LW, O'hara SM, Gross S. Analysis of circulating tumor cells, fragments, and debris. United States Patent 7863012. 2011.
-
- Asworth TR. A case of cancer in which cells similar to those in tumors were seen in the blood after death. Aust Med J. 1869;14:146–149.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

