Development of the UV spectrophotometric method of Olmesartan medoxomil in bulk drug and pharmaceutical formulation and stress degradation studies
- PMID: 23781428
- PMCID: PMC3658033
- DOI: 10.4103/2229-4708.81092
Development of the UV spectrophotometric method of Olmesartan medoxomil in bulk drug and pharmaceutical formulation and stress degradation studies
Abstract
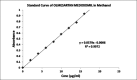
A simple, sensitive, specific, spectrophotometric method was developed for the detection of Olmesartan medoxomil (OLM) in bulk and pharmaceutical formulations. The optimum conditions for the analysis of the drug were established. OLM was subjected to stress degradation under different conditions recommended by the International Conference on Harmonization (ICH). The samples so generated were used for degradation studies using the developed method. The λmax of the OLM was found to be 257 nm. The method exhibited high sensitivity, with linearity, in the 2 to 20 μg/ml range. The lower limit of detection and the limit of quantification were found to be 1.012 μg/ml and 3.036 μg/ml, respectively. All the calibration curves demonstrated a linear relationship between the absorbance and concentration, with the correlation coefficient higher than 0.99. The regression equation of the curve was Y = 0.0579x + 0.0006. The precision of the method was found to be 40.043 ± 0.067 against the label claim of 40 mg. The percentage recovery was found to be 101.32 ± 0.452. The sample solution was stable for up to two hours. Hence, it could be concluded that the proposed method would be suitable for the analysis of OLM in bulk and pharmaceutical formulations.
Keywords: Estimation; Olmesartan medoxomil; spectroscopy; stress degradation study.
Conflict of interest statement
Figures
References
-
- Nagaoka A. OLM. New Cardiovascular Drugs. 1987:217–235.
-
- Wempe MF, Lightner JW, Zoeller EL, Rice PJ. Investigating OLM and OLMlinoleate metabolism: In vitro pig ear and mouse melanocyte studies. J Cos Dermat. 2009;8:63–73. - PubMed
-
- Wakabayashi H, Nakajima M, Yamato S, Shimada K. Determination of OLM in rat serum and brain by high-performance liquid chromatography using platinum catalyst reduction and electrochemical detection. J Chromato. 1992;573:154–7. - PubMed
-
- Rane VP, Patil KR, Shangshetti JN, Yeole RD, Shinde DB. Stability-indicating LC method for the determination of olmesartan in bulk drug and in pharmaceutical dosage form. Chromatographia. 2009;69:169–73.
-
- Vaidya VV, Roy SM, Yetal SM, Joshi SS, Parekh SS. LC–MS–MS determination of olmesartan in human plasma. Chromatographia. 2008;67:147–50.
LinkOut - more resources
Full Text Sources