Efficacy and safety of Privigen(®) in patients with chronic inflammatory demyelinating polyneuropathy: results of a prospective, single-arm, open-label Phase III study (the PRIMA study)
- PMID: 23781960
- PMCID: PMC3910165
- DOI: 10.1111/jns5.12017
Efficacy and safety of Privigen(®) in patients with chronic inflammatory demyelinating polyneuropathy: results of a prospective, single-arm, open-label Phase III study (the PRIMA study)
Abstract
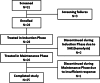
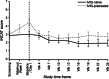
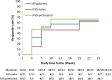
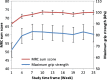
This prospective, multicenter, single-arm, open-label Phase III study aimed to evaluate the efficacy and safety of Privigen(®) (10% liquid human intravenous immunoglobulin [IVIG], stabilized with L-proline) in patients with chronic inflammatory demyelinating polyneuropathy (CIDP). Patients received one induction dose of Privigen (2 g/kg body weight [bw]) and up to seven maintenance doses (1 g/kg bw) at 3-week intervals. The primary efficacy endpoint was the responder rate at completion, defined as improvement of ≥1 point on the adjusted Inflammatory Neuropathy Cause and Treatment (INCAT) disability scale. The preset success criterion was the responder rate being ≥35%. Of the 31 screened patients, 28 patients were enrolled including 13 (46.4%) IVIG-pretreated patients. The overall responder rate at completion was 60.7% (95% confidence interval [CI]: 42.41%-76.43%). IVIG-pretreated patients demonstrated a higher responder rate than IVIG-naïve patients (76.9% vs. 46.7%). The median (25%-75% quantile) INCAT score improved from 3.5 (3.0-4.5) points at baseline to 2.5 (1.0-3.0) points at completion, as did the mean (standard deviation [SD]) maximum grip strength (66.7 [37.24] kPa vs. 80.9 [31.06] kPa) and the median Medical Research Council sum score (67.0 [61.5-72.0] points vs. 75.5 [71.5-79.5] points). Of 108 adverse events (AEs; 0.417 AEs per infusion), 95 AEs (88.0%) were mild or moderate in intensity and resolved by the end of study. Two serious AEs of hemolysis were reported that resolved after discontinuation of treatment. Thus, Privigen provided efficacious and well-tolerated induction and maintenance treatment in patients with CIDP.
© 2013 The Authors. Journal of the Peripheral Nervous System published by Wiley Periodicals, Inc. on behalf of Peripheral Nerve Society.
Figures

References
-
- Baxley A, Akhtari M. Hematologic toxicities associated with intravenous immunoglobulin therapy. Int Immunopharmacol. 2011;11:1663–1667. - PubMed
-
- Daw Z, Padmore R, Neurath D, Cober N, Tokessy M, Desjardins D, Olberg B, Tinmouth A, Giulivi A. Hemolytic transfusion reactions after administration of intravenous immune (gamma) globulin: a case series analysis. Transfusion. 2008;48:1598–1601. - PubMed
-
- Funfgeld EW. The vigorimeter: for measurement of the strength of the hand and simulation testing. Dtsch Med Wochenschr. 1966;91:2214–2216. - PubMed
-
- Hahn AF, Bolton CF, Zochodne D, Feasby TE. Intravenous immunoglobulin treatment in chronic inflammatory demyelinating polyneuropathy. A double-blind, placebo-controlled, cross-over study. Brain. 1996;119:1067–1077. - PubMed
-
- Hughes R, Bensa S, Willison H, Van den Bergh P, Comi G, Illa I, Nobile-Orazio E, van Doorn P, Dalakas M, Bojar M, Swan A Inflammatory Neuropathy Cause and Treatment (INCAT) Group. Randomized controlled trial of intravenous immunoglobulin versus oral prednisolone in chronic inflammatory demyelinating polyradiculoneuropathy. Ann Neurol. 2001;50:195–201. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

