Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma
- PMID: 23782157
- PMCID: PMC4513941
- DOI: 10.1056/NEJMoa1306220
Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma
Abstract
Background: Bruton's tyrosine kinase (BTK) is a mediator of the B-cell-receptor signaling pathway implicated in the pathogenesis of B-cell cancers. In a phase 1 study, ibrutinib, a BTK inhibitor, showed antitumor activity in several types of non-Hodgkin's lymphoma, including mantle-cell lymphoma.
Methods: In this phase 2 study, we investigated oral ibrutinib, at a daily dose of 560 mg, in 111 patients with relapsed or refractory mantle-cell lymphoma. Patients were enrolled into two groups: those who had previously received at least 2 cycles of bortezomib therapy and those who had received less than 2 complete cycles of bortezomib or had received no prior bortezomib therapy. The primary end point was the overall response rate. Secondary end points were duration of response, progression-free survival, overall survival, and safety.
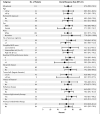
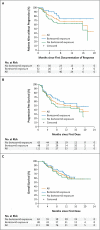
Results: The median age was 68 years, and 86% of patients had intermediate-risk or high-risk mantle-cell lymphoma according to clinical prognostic factors. Patients had received a median of three prior therapies. The most common treatment-related adverse events were mild or moderate diarrhea, fatigue, and nausea. Grade 3 or higher hematologic events were infrequent and included neutropenia (in 16% of patients), thrombocytopenia (in 11%), and anemia (in 10%). A response rate of 68% (75 patients) was observed, with a complete response rate of 21% and a partial response rate of 47%; prior treatment with bortezomib had no effect on the response rate. With an estimated median follow-up of 15.3 months, the estimated median response duration was 17.5 months (95% confidence interval [CI], 15.8 to not reached), the estimated median progression-free survival was 13.9 months (95% CI, 7.0 to not reached), and the median overall survival was not reached. The estimated rate of overall survival was 58% at 18 months.
Conclusions: Ibrutinib shows durable single-agent efficacy in relapsed or refractory mantle-cell lymphoma. (Funded by Pharmacyclics and others; ClinicalTrials.gov number, NCT01236391.)
Figures
Comment in
-
Toward new treatments for mantle-cell lymphoma?N Engl J Med. 2013 Aug 8;369(6):571-2. doi: 10.1056/NEJMe1307596. N Engl J Med. 2013. PMID: 23924008 No abstract available.
References
-
- A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma: the Non-Hodgkin's Lymphoma Classification Project. Blood. 1997;89:3909–18. - PubMed
-
-
Romaguera JE, Fayad LE, Feng L, et al. Ten-year follow-up after intense chemoimmunotherapy with rituximab-hyperCVAD alternating with rituximab-high dose methotrexate/cytarabine (R-MA) and without stem cell transplantation in patients with untreated aggressive mantle cell lymphoma. Br J Haematol. 2010;150:200–8. [Erratum, Br J Haematol 2010;151:111.]
-
-
- Wiestner A. Targeting B-cell receptor signaling for anticancer therapy: the Bruton's tyrosine kinase inhibitor ibrutinib induces impressive responses in B-cell malignancies. J Clin Oncol. 2013;31:128–30. - PubMed
-
- Kenkre VP, Kahl BS. The future of B-cell lymphoma therapy: the B-cell receptor and its downstream pathways. Curr Hematol Malig Rep. 2012;7:216–20. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
