Preventing the onset of major depression based on the level and profile of risk of primary care attendees: protocol of a cluster randomised trial (the predictD-CCRT study)
- PMID: 23782553
- PMCID: PMC3698147
- DOI: 10.1186/1471-244X-13-171
Preventing the onset of major depression based on the level and profile of risk of primary care attendees: protocol of a cluster randomised trial (the predictD-CCRT study)
Abstract
Background: The 'predictD algorithm' provides an estimate of the level and profile of risk of the onset of major depression in primary care attendees. This gives us the opportunity to develop interventions to prevent depression in a personalized way. We aim to evaluate the effectiveness, cost-effectiveness and cost-utility of a new intervention, personalized and implemented by family physicians (FPs), to prevent the onset of episodes of major depression.
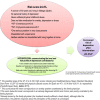
Methods/design: This is a multicenter randomized controlled trial (RCT), with cluster assignment by health center and two parallel arms. Two interventions will be applied by FPs, usual care versus the new intervention predictD-CCRT. The latter has four components: a training workshop for FPs; communicating the level and profile of risk of depression; building up a tailored bio-psycho-family-social intervention by FPs to prevent depression; offering a booklet to prevent depression; and activating and empowering patients. We will recruit a systematic random sample of 3286 non-depressed adult patients (1643 in each trial arm), nested in 140 FPs and 70 health centers from 7 Spanish cities. All patients will be evaluated at baseline, 6, 12 and 18 months. The level and profile of risk of depression will be communicated to patients by the FPs in the intervention practices at baseline, 6 and 12 months. Our primary outcome will be the cumulative incidence of major depression (measured by CIDI each 6 months) over 18 months of follow-up. Secondary outcomes will be health-related quality of life (SF-12 and EuroQol), and measurements of cost-effectiveness and cost-utility. The inferences will be made at patient level. We shall undertake an intention-to-treat effectiveness analysis and will handle missing data using multiple imputations. We will perform multi-level logistic regressions and will adjust for the probability of the onset of major depression at 12 months measured at baseline as well as for unbalanced variables if appropriate. The economic evaluation will be approached from two perspectives, societal and health system.
Discussion: To our knowledge, this will be the first RCT of universal primary prevention for depression in adults and the first to test a personalized intervention implemented by FPs. We discuss possible biases as well as other limitations.
Trial registration: ClinicalTrials.gov identifier: NCT01151982.
Figures

References
-
- The ESEMeD⁄MHEDEA 2000 Investigators. Prevalence of mental disorders in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand. 2004;109(Suppl. 420):21–27. - PubMed
-
- King M, Nazareth I, Levy G, Walker C, Morris R, Weich S, Bellón JA, Moreno B, Svab I, Rotar D, Rifel J, Maaroos HI, Aluoja A, Kalda R, Neeleman J, Geerlings MI, Xavier M, de Almeida MC, Correa B, Torres-González F. Prevalence of common mental disorders in general practice attendees across Europe. Br J Psychiatry. 2008;192:362–367. doi: 10.1192/bjp.bp.107.039966. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

