Brain-specific angiogenesis inhibitor-1 signaling, regulation, and enrichment in the postsynaptic density
- PMID: 23782696
- PMCID: PMC3829316
- DOI: 10.1074/jbc.M113.489757
Brain-specific angiogenesis inhibitor-1 signaling, regulation, and enrichment in the postsynaptic density
Abstract
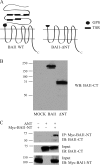
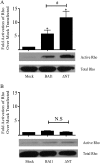
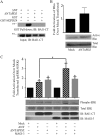
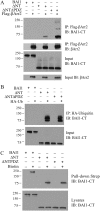
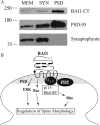
Brain-specific angiogenesis inhibitor-1 (BAI1) is an adhesion G protein-coupled receptor that has been studied primarily for its anti-angiogenic and anti-tumorigenic properties. We found that overexpression of BAI1 results in activation of the Rho pathway via a Gα(12/13)-dependent mechanism, with truncation of the BAI1 N terminus resulting in a dramatic enhancement in receptor signaling. This constitutive activity of the truncated BAI1 mutant also resulted in enhanced downstream phosphorylation of ERK as well as increased receptor association with β-arrestin2 and increased ubiquitination of the receptor. To gain insights into the regulation of BAI1 signaling, we screened the C terminus of BAI1 against a proteomic array of PDZ domains to identify novel interacting partners. These screens revealed that the BAI1 C terminus interacts with a variety of PDZ domains from synaptic proteins, including MAGI-3. Removal of the BAI1 PDZ-binding motif resulted in attenuation of receptor signaling to Rho but had no effect on ERK activation. Conversely, co-expression with MAGI-3 was found to potentiate signaling to ERK by constitutively active BAI1 in a manner that was dependent on the PDZ-binding motif of the receptor. Biochemical fractionation studies revealed that BAI1 is highly enriched in post-synaptic density fractions, a finding consistent with our observations that BAI1 can interact with PDZ proteins known to be concentrated in the post-synaptic density. These findings demonstrate that BAI1 is a synaptic receptor that can activate both the Rho and ERK pathways, with the N-terminal and C-terminal regions of the receptor playing key roles in the regulation of BAI1 signaling activity.
Keywords: 7-Helix Receptor; Adhesion; Arrestin; Brain; G Protein-coupled Receptors (GPCR); G Proteins; Rho GTPases; Scaffold Proteins; Signaling; Synapses.
Figures

References
-
- Yona S., Lin H. H., Siu W. O., Gordon S., Stacey M. (2008) Adhesion-GPCRs: emerging roles for novel receptors. Trends Biochem. Sci. 33, 491–500 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

