Modulating the intrinsic disorder in the cytoplasmic domain alters the biological activity of the N-methyl-D-aspartate-sensitive glutamate receptor
- PMID: 23782697
- PMCID: PMC3829338
- DOI: 10.1074/jbc.M113.477810
Modulating the intrinsic disorder in the cytoplasmic domain alters the biological activity of the N-methyl-D-aspartate-sensitive glutamate receptor
Abstract
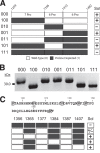
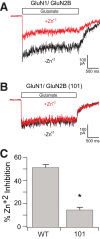
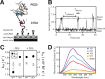
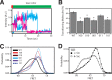
The NMDA-sensitive glutamate receptor is a ligand-gated ion channel that mediates excitatory synaptic transmission in the nervous system. Extracellular zinc allosterically regulates the NMDA receptor by binding to the extracellular N-terminal domain, which inhibits channel gating. Phosphorylation of the intrinsically disordered intracellular C-terminal domain alleviates inhibition by extracellular zinc. The mechanism for this functional effect is largely unknown. Proline is a hallmark of intrinsic disorder, so we used proline mutagenesis to modulate disorder in the cytoplasmic domain. Proline depletion selectively uncoupled zinc inhibition with little effect on receptor biogenesis, surface trafficking, or ligand-activated gating. Proline depletion also reduced the affinity for a PDZ domain involved in synaptic trafficking and affected small molecule binding. To understand the origin of these phenomena, we used single molecule fluorescence and ensemble biophysical methods to characterize the structural effects of proline mutagenesis. Proline depletion did not eliminate intrinsic disorder, but the underlying conformational dynamics were changed. Thus, we altered the form of intrinsic disorder, which appears sufficient to affect the biological activity. These findings suggest that conformational dynamics within the intrinsically disordered cytoplasmic domain are important for the allosteric regulation of NMDA receptor gating.
Keywords: Electrophysiology; Fluorescence Resonance Energy Transfer (FRET); Glutamate Receptors Ionotropic (AMPA, NMDA); Intrinsically Disordered Proteins; Single Molecule Biophysics.
Figures


References
-
- Lau C. G., Zukin R. S. (2007) NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat. Rev. Neurosci. 8, 413–426 - PubMed
-
- Ryan T. J., Kopanitsa M. V., Indersmitten T., Nithianantharajah J., Afinowi N. O., Pettit C., Stanford L. E., Sprengel R., Saksida L. M., Bussey T. J., O'Dell T. J., Grant S. G., Komiyama N. H. (2013) Evolution of GluN2A/B cytoplasmic domains diversified vertebrate synaptic plasticity and behavior. Nat. Neurosci. 16, 25–32 - PMC - PubMed
-
- Sprengel R., Suchanek B., Amico C., Brusa R., Burnashev N., Rozov A., Hvalby O., Jensen V., Paulsen O., Andersen P., Kim J. J., Thompson R. F., Sun W., Webster L. C., Grant S. G., Eilers J., Konnerth A., Li J., McNamara J. O., Seeburg P. H. (1998) Importance of the intracellular domain of NR2 subunits for NMDA receptor function in vivo. Cell 92, 279–289 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

