Genistein interferes with SDF-1- and HIV-mediated actin dynamics and inhibits HIV infection of resting CD4 T cells
- PMID: 23782904
- PMCID: PMC3693989
- DOI: 10.1186/1742-4690-10-62
Genistein interferes with SDF-1- and HIV-mediated actin dynamics and inhibits HIV infection of resting CD4 T cells
Abstract
Background: Binding of HIV to the chemokine coreceptor CXCR4 mediates viral fusion and signal transduction that promotes actin dynamics critical for HIV infection of blood resting CD4 T cells. It has been suggested that this gp120-mediated actin activity resembles the chemotactic actin dynamics mediated by chemokines such as SDF-1. To determine whether inhibiting SDF-1-mediated chemotactic activity can also inhibit HIV infection, we screened several inhibitors known to reduce SDF-1-mediated chemotaxis of T cells.
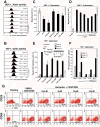
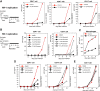
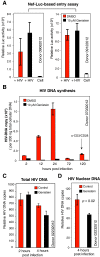
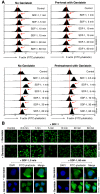
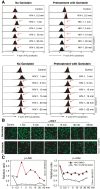
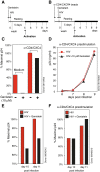
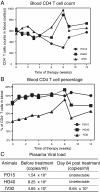
Results: We found that a tyrosine kinase inhibitor, genistein, inhibited both SDF-1-mediated chemotaxis and HIV infection of resting CD4 T cells. Genistein was also found to interfere with SDF-1- and HIV-mediated actin dynamics in CD4 T cells. This reduction in actin activity correlates with genistein-mediated inhibition of viral DNA accumulation in resting CD4 T cells. In addition, we also tested two other tyrosine kinase inhibitors, sunitinib and AG1478. Sunitinib, but not AG1478, inhibited HIV infection of resting CD4 T cells. We further tested the safety of genistein in 3 Chinese rhesus macaques (Macaca mulatta), and each animal was given a monotherapy of genistein at 10 mg/kg orally for 12 weeks. No adverse drug effects were observed in these animals.
Conclusions: Our results suggest that novel therapeutic strategies can be developed based on targeting cellular proteins involved in HIV-dependent signaling. This approach can interfere with HIV-mediated actin dynamics and inhibit HIV infection.
Figures
Similar articles
-
HIV gp120 is an aberrant chemoattractant for blood resting CD4 T cells.Curr HIV Res. 2012 Dec;10(8):636-42. doi: 10.2174/157016212803901365. Curr HIV Res. 2012. PMID: 22954308
-
V3 loop-determined coreceptor preference dictates the dynamics of CD4+-T-cell loss in simian-human immunodeficiency virus-infected macaques.J Virol. 2005 Oct;79(19):12296-303. doi: 10.1128/JVI.79.19.12296-12303.2005. J Virol. 2005. PMID: 16160156 Free PMC article.
-
LIM kinase 1 modulates cortical actin and CXCR4 cycling and is activated by HIV-1 to initiate viral infection.J Biol Chem. 2011 Apr 8;286(14):12554-64. doi: 10.1074/jbc.M110.182238. Epub 2011 Feb 14. J Biol Chem. 2011. PMID: 21321123 Free PMC article.
-
HIV Persistence in Adipose Tissue Reservoirs.Curr HIV/AIDS Rep. 2018 Feb;15(1):60-71. doi: 10.1007/s11904-018-0378-z. Curr HIV/AIDS Rep. 2018. PMID: 29423731 Free PMC article. Review.
-
Understanding the basis of CD4(+) T-cell depletion in macaques infected by a simian-human immunodeficiency virus.Vaccine. 2002 May 6;20(15):1934-7. doi: 10.1016/s0264-410x(02)00072-5. Vaccine. 2002. PMID: 11983249 Review.
Cited by
-
Osteoimmune Properties of Mesoporous Bioactive Nanospheres: A Study on T Helper Lymphocytes.Nanomaterials (Basel). 2023 Jul 26;13(15):2183. doi: 10.3390/nano13152183. Nanomaterials (Basel). 2023. PMID: 37570501 Free PMC article.
-
Flavonoids: promising natural compounds against viral infections.Arch Virol. 2017 Sep;162(9):2539-2551. doi: 10.1007/s00705-017-3417-y. Epub 2017 May 25. Arch Virol. 2017. PMID: 28547385 Free PMC article. Review.
-
Prestimulation of CD2 confers resistance to HIV-1 latent infection in blood resting CD4 T cells.iScience. 2021 Oct 16;24(11):103305. doi: 10.1016/j.isci.2021.103305. eCollection 2021 Nov 19. iScience. 2021. PMID: 34765923 Free PMC article.
-
Drug repurposing for new, efficient, broad spectrum antivirals.Virus Res. 2019 Apr 15;264:22-31. doi: 10.1016/j.virusres.2019.02.011. Epub 2019 Feb 19. Virus Res. 2019. PMID: 30794895 Free PMC article. Review.
-
Novel anti-HIV therapeutics targeting chemokine receptors and actin regulatory pathways.Immunol Rev. 2013 Nov;256(1):300-12. doi: 10.1111/imr.12106. Immunol Rev. 2013. PMID: 24117829 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

