Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon α-2a
- PMID: 23782935
- PMCID: PMC3739035
- DOI: 10.1182/blood-2012-07-442012
Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon α-2a
Abstract
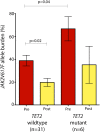
Pegylated interferon α-2a (PEG-IFN-α-2a) has previously been shown to induce hematologic and molecular responses in patients with polycythemia vera (PV) or essential thrombocythemia (ET). Here we present a follow-up of a phase 2 trial with PEG-IFN-α-2a treatment in 43 PV and 40 ET patients with detailed molecular analysis. After a median follow-up of 42 months, complete hematologic response was achieved in 76% of patients with PV and 77% of those with ET. This was accompanied by complete molecular response (CMR) (ie, undetectable JAK2V617F) in 18% and 17%, of PV and ET patients, respectively. Serial sequencing of TET2, ASXL1, EZH2, DNMT3A, and IDH1/2 revealed that patients failing to achieve CMR had a higher frequency of mutations outside the Janus kinase-signal transducer and activator of transcription pathway and were more likely to acquire new mutations during therapy. Patients with both JAK2V617F and TET2 mutations at therapy onset had a higher JAK2V617F mutant allele burden and a less significant reduction in JAK2V617F allele burden compared with JAK2 mutant/TET2 wild-type patients. These data demonstrate that PEG-IFN-α-2a induces sustained CMR in a subset of PV or ET patients, and that genotypic context may influence clinical and molecular response to PEG-IFN-α-2a.
Figures


References
-
- Baxter EJ, Scott LM, Campbell PJ, et al. Cancer Genome Project. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–1061. - PubMed
-
- James C, Ugo V, Le Couédic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–1148. - PubMed
-
- Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–1790. - PubMed
-
- Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

