Mammary-specific ablation of the calcium-sensing receptor during lactation alters maternal calcium metabolism, milk calcium transport, and neonatal calcium accrual
- PMID: 23782944
- PMCID: PMC3749485
- DOI: 10.1210/en.2012-2195
Mammary-specific ablation of the calcium-sensing receptor during lactation alters maternal calcium metabolism, milk calcium transport, and neonatal calcium accrual
Abstract
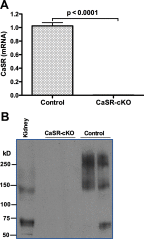
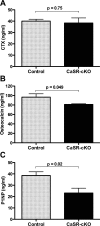
To meet the demands for milk calcium, the lactating mother adjusts systemic calcium and bone metabolism by increasing dietary calcium intake, increasing bone resorption, and reducing renal calcium excretion. As part of this adaptation, the lactating mammary gland secretes PTHrP into the maternal circulation to increase bone turnover and mobilize skeletal calcium stores. Previous data have suggested that, during lactation, the breast relies on the calcium-sensing receptor (CaSR) to coordinate PTHrP secretion and milk calcium transport with calcium availability. To test this idea genetically, we bred BLG-Cre mice with CaSR-floxed mice to ablate the CaSR specifically from mammary epithelial cells only at the onset of lactation (CaSR-cKO mice). Loss of the CaSR in the lactating mammary gland did not disrupt alveolar differentiation or milk production. However, it did increase the secretion of PTHrP into milk and decreased the transport of calcium from the circulation into milk. CaSR-cKO mice did not show accelerated bone resorption, but they did have a decrease in bone formation. Loss of the mammary gland CaSR resulted in hypercalcemia, decreased PTH secretion, and increased renal calcium excretion in lactating mothers. Finally, loss of the mammary gland CaSR resulted in decreased calcium accrual by suckling neonates, likely due to the combination of increased milk PTHrP and decreased milk calcium. These results demonstrate that the mammary gland CaSR coordinates maternal bone and calcium metabolism, calcium transport into milk, and neonatal calcium accrual during lactation.
Figures





References
-
- Kovacs CS. Calcium and bone metabolism during pregnancy and lactation. J Mamm Gland Biol Neoplasia. 2005;10(2):105–118 - PubMed
-
- Kovacs CS, Kronenberg HM. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev. 1997;18(6):832–872 - PubMed
-
- VanHouten J. Maternal calcium and bone metabolism during lactation. Curr Opin Endocrinol Diabetes. 2005;12:477–482
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG021353/AG/NIA NIH HHS/United States
- AR055924/AR/NIAMS NIH HHS/United States
- P30DK045735/DK/NIDDK NIH HHS/United States
- P30 AR046032/AR/NIAMS NIH HHS/United States
- I01 BX001066/BX/BLRD VA/United States
- R01 AR055924/AR/NIAMS NIH HHS/United States
- R01 DK054793/DK/NIDDK NIH HHS/United States
- P30AR46032/AR/NIAMS NIH HHS/United States
- CA153702/CA/NCI NIH HHS/United States
- DK054793/DK/NIDDK NIH HHS/United States
- R01 CA153702/CA/NCI NIH HHS/United States
- R01 AR050023/AR/NIAMS NIH HHS/United States
- AG21353/AG/NIA NIH HHS/United States
- R01 AR038386/AR/NIAMS NIH HHS/United States
- R01 DK055501/DK/NIDDK NIH HHS/United States
- DK55501/DK/NIDDK NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
- AR050023/AR/NIAMS NIH HHS/United States
- R01 DK077565/DK/NIDDK NIH HHS/United States
- DK077565/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

