Construction of the coding sequence of the transcription variant 2 of the human Renalase gene and its expression in the prokaryotic system
- PMID: 23783275
- PMCID: PMC3709811
- DOI: 10.3390/ijms140612764
Construction of the coding sequence of the transcription variant 2 of the human Renalase gene and its expression in the prokaryotic system
Abstract
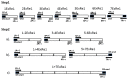
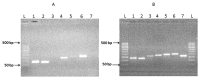
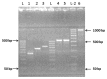
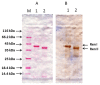
Renalase is a recently discovered protein, involved in regulation of blood pressure in humans and animals. Although several splice variants of human renalase mRNA transcripts have been recognized, only one protein product, hRenalase1, has been found so far. In this study, we have used polymerase chain reaction (PCR)-based amplification of individual exons of the renalase gene and their joining for construction of full-length hRenalase2 coding sequence followed by expression of hRenalase2 as a polyHis recombinant protein in Escherichia coli cells. To date this is the first report on synthesis and purification of hRenalase2. Applicability of this approach was verified by constructing hRenalase1 coding sequence, its sequencing and expression in E. coli cells. hRenalase1 was used for generation of polyclonal antiserum in sheep. Western blot analysis has shown that polyclonal anti-renalase1 antibodies effectively interact with the hRenalase2 protein. The latter suggests that some functions and expression patterns of hRenalase1 documented by antibody-based data may be attributed to the presence of hRenalase2. The realized approach may be also used for construction of coding sequences of various (especially weakly expressible) genes, their transcript variants, etc.
Figures


References
-
- Baroni S., Milani M., Pandini V., Pavesi G., Horner D., Aliverti A. Is renalase a novel player in catecholaminergic signaling? The mystery of the catalytic activity of an intriguing new flavoenzyme. Curr. Pharm. Des. 2013;19:2540–2551. - PubMed
-
- Desir G.V., Wang L., Peixoto A.J. Human renalase: A review of its biology, function, and implications for hypertension. J. Am. Soc. Hypertens. 2012;6:417–426. - PubMed
-
- Malyszko J., Malyszko J.S., Rysz J., Mysliwiec M., Tesar V., Levin-Iaina N., Banach M. Renalase, Hypertension, and Kidney—The Discussion Continues. Angiology. 2013;64:181–187. - PubMed
-
- Medvedev A.E., Veselovsky A.V., Fedchenko V.I. Renalase, a new secretory enzyme responsible for selective degradation of catecholamines: Achievements and unsolved problems. Biochemistry. 2010;75:951–958. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

