The PAK system links Rho GTPase signaling to thrombin-mediated platelet activation
- PMID: 23784547
- PMCID: PMC3761148
- DOI: 10.1152/ajpcell.00418.2012
The PAK system links Rho GTPase signaling to thrombin-mediated platelet activation
Abstract
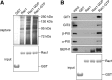
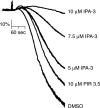
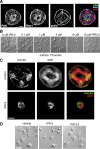
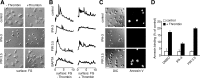
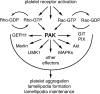
Regulation of the platelet actin cytoskeleton by the Rho family of small GTPases is essential for the proper maintenance of hemostasis. However, little is known about how intracellular platelet activation from Rho GTPase family members, including Rac, Cdc42, and Rho, translate into changes in platelet actin structures. To better understand how Rho family GTPases coordinate platelet activation, we identified platelet proteins associated with Rac1, a Rho GTPase family member, and actin regulatory protein essential for platelet hemostatic function. Mass spectrometry analysis revealed that upon platelet activation with thrombin, Rac1 associates with a set of effectors of the p21-activated kinases (PAKs), including GIT1, βPIX, and guanine nucleotide exchange factor GEFH1. Platelet activation by thrombin triggered the PAK-dependent phosphorylation of GIT1, GEFH1, and other PAK effectors, including LIMK1 and Merlin. PAK was also required for the thrombin-mediated activation of the MEK/ERK pathway, Akt, calcium signaling, and phosphatidylserine (PS) exposure. Inhibition of PAK signaling prevented thrombin-induced platelet aggregation and blocked platelet focal adhesion and lamellipodia formation in response to thrombin. Together, these results demonstrate that the PAK signaling system is a key orchestrator of platelet actin dynamics, linking Rho GTPase activation downstream of thrombin stimulation to PAK effector function, MAP kinase activation, calcium signaling, and PS exposure in platelets.
Keywords: PAK; Rac1; Rho GTPases; actin; platelets.
Figures


References
-
- Arias-Romero LE, Chernoff J. A tale of two Paks. Biol Cell 100: 97–108, 2008 - PubMed
-
- Aslan JE, Itakura A, Gertz JM, McCarty OJ. Platelet shape change and spreading. Methods Mol Biol 788: 91–100, 2012 - PubMed
-
- Aslan JE, Itakura A, Haley KM, Tormoen GW, Loren CP, Baker SM, Pang J, Chernoff J, McCarty OJ. p21 activated kinase signaling coordinates glycoprotein receptor vi-mediated platelet aggregation, lamellipodia formation, and aggregate stability under shear. Arterioscler Thromb Vasc Biol 33: 1544–1551, 2013 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

