Gβγ-independent recruitment of G-protein coupled receptor kinase 2 drives tumor necrosis factor α-induced cardiac β-adrenergic receptor dysfunction
- PMID: 23785004
- PMCID: PMC3874808
- DOI: 10.1161/CIRCULATIONAHA.113.003183
Gβγ-independent recruitment of G-protein coupled receptor kinase 2 drives tumor necrosis factor α-induced cardiac β-adrenergic receptor dysfunction
Abstract
Background: Proinflammatory cytokine tumor necrosis factor-α (TNFα) induces β-adrenergic receptor (βAR) desensitization, but mechanisms proximal to the receptor in contributing to cardiac dysfunction are not known.
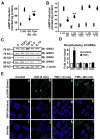
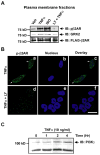
Methods and results: Two different proinflammatory transgenic mouse models with cardiac overexpression of myotrophin (a prohypertrophic molecule) or TNFα showed that TNFα alone is sufficient to mediate βAR desensitization as measured by cardiac adenylyl cyclase activity. M-mode echocardiography in these mouse models showed cardiac dysfunction paralleling βAR desensitization independent of sympathetic overdrive. TNFα-mediated βAR desensitization that precedes cardiac dysfunction is associated with selective upregulation of G-protein coupled receptor kinase 2 (GRK2) in both mouse models. In vitro studies in β2AR-overexpressing human embryonic kidney 293 cells showed significant βAR desensitization, GRK2 upregulation, and recruitment to the βAR complex following TNFα. Interestingly, inhibition of phosphoinositide 3-kinase abolished GRK2-mediated βAR phosphorylation and GRK2 recruitment on TNFα. Furthermore, TNFα-mediated βAR phosphorylation was not blocked with βAR antagonist propranolol. Additionally, TNFα administration in transgenic mice with cardiac overexpression of Gβγ-sequestering peptide βARK-ct could not prevent βAR desensitization or cardiac dysfunction showing that GRK2 recruitment to the βAR is Gβγ independent. Small interfering RNA knockdown of GRK2 resulted in the loss of TNFα-mediated βAR phosphorylation. Consistently, cardiomyocytes from mice with cardiac-specific GRK2 ablation normalized the TNFα-mediated loss in contractility, showing that TNFα-induced βAR desensitization is GRK2 dependent.
Conclusions: TNFα-induced βAR desensitization is mediated by GRK2 and is independent of Gβγ, uncovering a hitherto unknown cross-talk between TNFα and βAR function, providing the underpinnings of inflammation-mediated cardiac dysfunction.
Keywords: G-protein coupled receptor kinase 2; heart failure; inflammation; phosphoinositide 3-kinase; tumor necrosis factor-α; tumor necrosis factor-α receptor 2; β-adrenergic receptor.
Conflict of interest statement
Figures






Similar articles
-
Mdm2 regulates cardiac contractility by inhibiting GRK2-mediated desensitization of β-adrenergic receptor signaling.JCI Insight. 2017 Sep 7;2(17):e95998. doi: 10.1172/jci.insight.95998. eCollection 2017 Sep 7. JCI Insight. 2017. PMID: 28878120 Free PMC article.
-
Gi-biased β2AR signaling links GRK2 upregulation to heart failure.Circ Res. 2012 Jan 20;110(2):265-74. doi: 10.1161/CIRCRESAHA.111.253260. Epub 2011 Dec 15. Circ Res. 2012. PMID: 22179058 Free PMC article.
-
G protein-coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure.Circ Res. 2008 Aug 15;103(4):413-22. doi: 10.1161/CIRCRESAHA.107.168336. Epub 2008 Jul 17. Circ Res. 2008. PMID: 18635825 Free PMC article.
-
G protein-coupled receptor kinase 2: a link between myocardial contractile function and cardiac metabolism.Circ Res. 2014 May 9;114(10):1661-70. doi: 10.1161/CIRCRESAHA.114.300513. Circ Res. 2014. PMID: 24812353 Free PMC article. Review.
-
Targeting myocardial beta-adrenergic receptor signaling and calcium cycling for heart failure gene therapy.J Card Fail. 2007 Jun;13(5):401-14. doi: 10.1016/j.cardfail.2007.01.003. J Card Fail. 2007. PMID: 17602988 Review.
Cited by
-
Tumor Necrosis Factor-α in Heart Failure: an Updated Review.Curr Cardiol Rep. 2018 Sep 26;20(11):117. doi: 10.1007/s11886-018-1067-7. Curr Cardiol Rep. 2018. PMID: 30259192 Free PMC article. Review.
-
Viruses in the Heart: Direct and Indirect Routes to Myocarditis and Heart Failure.Viruses. 2021 Sep 24;13(10):1924. doi: 10.3390/v13101924. Viruses. 2021. PMID: 34696354 Free PMC article. Review.
-
Decreased FAM13B Expression Increases Atrial Fibrillation Susceptibility by Regulating Sodium Current and Calcium Handling.JACC Basic Transl Sci. 2023 Jul 26;8(10):1357-1378. doi: 10.1016/j.jacbts.2023.05.009. eCollection 2023 Oct. JACC Basic Transl Sci. 2023. PMID: 38094680 Free PMC article.
-
Myocardial 123I-mIBG scintigraphy in relation to markers of inflammation and long-term clinical outcome in patients with stable chronic heart failure.J Nucl Cardiol. 2018 Jun;25(3):845-853. doi: 10.1007/s12350-016-0697-7. Epub 2016 Nov 17. J Nucl Cardiol. 2018. PMID: 27858345 Free PMC article.
-
Proinflammatory Cytokines Mediate GPCR Dysfunction.J Cardiovasc Pharmacol. 2017 Aug;70(2):61-73. doi: 10.1097/FJC.0000000000000456. J Cardiovasc Pharmacol. 2017. PMID: 28763371 Free PMC article. Review.
References
-
- Prabhu SD. Cytokine-induced modulation of cardiac function. Circ Res. 2004;95:1140–1153. - PubMed
-
- Amadou A, Nawrocki A, Best-Belpomme M, Pavoine C, Pecker F. Arachidonic acid mediates dual effect of tnf-alpha on ca2+ transients and contraction of adult rat cardiomyocytes. Am J Physiol Cell Physiol. 2002;282:C1339–1347. - PubMed
-
- Grandel U, Fink L, Blum A, Heep M, Buerke M, Kraemer HJ, Mayer K, Bohle RM, Seeger W, Grimminger F, Sibelius U. Endotoxin-induced myocardial tumor necrosis factor-alpha synthesis depresses contractility of isolated rat hearts: Evidence for a role of sphingosine and cyclooxygenase-2-derived thromboxane production. Circulation. 2000;102:2758–2764. - PubMed
-
- Stein B, Frank P, Schmitz W, Scholz H, Thoenes M. Endotoxin and cytokines induce direct cardiodepressive effects in mammalian cardiomyocytes via induction of nitric oxide synthase. J Mol Cell Cardiol. 1996;28:1631–1639. - PubMed
-
- Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

