Silver enhances antibiotic activity against gram-negative bacteria
- PMID: 23785037
- PMCID: PMC3771099
- DOI: 10.1126/scitranslmed.3006276
Silver enhances antibiotic activity against gram-negative bacteria
Abstract
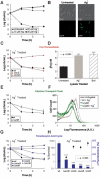
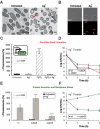
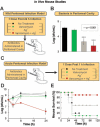
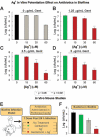
A declining pipeline of clinically useful antibiotics has made it imperative to develop more effective antimicrobial therapies, particularly against difficult-to-treat Gram-negative pathogens. Silver has been used as an antimicrobial since antiquity, yet its mechanism of action remains unclear. We show that silver disrupts multiple bacterial cellular processes, including disulfide bond formation, metabolism, and iron homeostasis. These changes lead to increased production of reactive oxygen species and increased membrane permeability of Gram-negative bacteria that can potentiate the activity of a broad range of antibiotics against Gram-negative bacteria in different metabolic states, as well as restore antibiotic susceptibility to a resistant bacterial strain. We show both in vitro and in a mouse model of urinary tract infection that the ability of silver to induce oxidative stress can be harnessed to potentiate antibiotic activity. Additionally, we demonstrate in vitro and in two different mouse models of peritonitis that silver sensitizes Gram-negative bacteria to the Gram-positive-specific antibiotic vancomycin, thereby expanding the antibacterial spectrum of this drug. Finally, we used silver and antibiotic combinations in vitro to eradicate bacterial persister cells, and show both in vitro and in a mouse biofilm infection model that silver can enhance antibacterial action against bacteria that produce biofilms. This work shows that silver can be used to enhance the action of existing antibiotics against Gram-negative bacteria, thus strengthening the antibiotic arsenal for fighting bacterial infections.
Figures



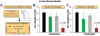

References
-
- Taubes G. The bacteria fight back. Science. 2008;321:356. - PubMed
-
- Li J, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect. Dis. 2006;6:589. - PubMed
-
- Pages JM, James CE, Winterhalter M. The porin and the permeating antibiotic: a selective diffusion barrier in Gram-negative bacteria. Nature Reviews Microbiology. 2008;6:893. - PubMed
-
- Li J, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infectious Diseases. 2006;6:589. - PubMed
-
- Falagas ME, Kasiakou SK. Colistin: the revival of polymixins for the management of multidrug-resistant Gram-negative bacterial infections. Clinical Infectious Diseases. 2005;40:1333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

