Baseline 18F-FDG PET/CT parameters as imaging biomarkers of overall survival in castrate-resistant metastatic prostate cancer
- PMID: 23785174
- PMCID: PMC3783857
- DOI: 10.2967/jnumed.112.114116
Baseline 18F-FDG PET/CT parameters as imaging biomarkers of overall survival in castrate-resistant metastatic prostate cancer
Abstract
The aim of this prospective investigation was to assess the association of parameters derived from baseline (18)F-FDG PET/CT with overall survival (OS) in men with castrate-resistant metastatic prostate cancer.
Methods: Eighty-seven men with castrate-resistant metastatic prostate cancer underwent (18)F-FDG PET/CT and were followed prospectively for OS. Median follow-up in patients who were alive was 22.2 mo (range, 1.6-62.5 mo). OS was defined as the time between the PET/CT imaging or the start of chemotherapy, whichever was later, and death, with patients who were alive censored at the last follow-up date. PET parameters included maximum standardized uptake value (SUV(max)) of the most active lesion, sum of SUV(max), and average SUV(max) of all metabolically active lesions, after subtraction of patient-specific background-liver average SUV. Comparison of OS was based on univariate and multivariable Cox regression analyses of continuous PET parameters adjusted for standard clinical parameters (age, serum prostate-specific antigen level, alkaline phosphatase, use of pain medication, prior chemotherapy, and Gleason score at initial diagnosis). Survival curves based on Kaplan-Meier estimates are presented.
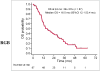
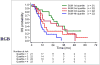
Results: Among the 87 patients, 61 were dead at the time of last follow-up. Median OS was 16.5 mo (95% confidence interval [CI], 12.1-23.4 mo), and the OS probability at 24 mo was 39% ± 6%. For the univariate analysis, the hazard ratios associated with each unit increase were 1.01 (95% CI, 1.006-1.02) for sum of SUV(max) (P = 0.002), 1.11 (95% CI, 1.03-1.18) for maximum SUV(max) (P = 0.010), and 1.13 (95% CI, 0.99-1.30) for average SUV(max) (P = 0.095). For the multivariable analysis adjusting for relevant clinical parameters, the continuous parameter sum of SUV(max) remained significant (P = 0.053), with a hazard ratio of 1.01 (95% CI, 1.001-1.02). When sum of SUV(max) was grouped into quartile ranges, there was poorer survival probability for the patients in the fourth-quartile range than for those in the first-quartile range, with a univariate hazard ratio of 3.8 (95% CI, 1.8-7.9).
Conclusion: Sum of SUV(max) derived from (18)F-FDG PET/CT contributes independent prognostic information on OS in men with castrate-resistant metastatic prostate cancer, and this information may be useful in assessing the comparative effectiveness of various conventional and emerging treatment strategies.
Trial registration: ClinicalTrials.gov NCT00282906.
Keywords: 18F-FDG; cancer; castrate-resistant; prostate; survival.
Figures


References
-
- SEER stat fact sheets: prostate. National Cancer Institute; [Accessed 5 June 2013]. Web site. http://seer.cancer.gov/statfacts/html/prost.html.
-
- Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the PSA Working Group. J Clin Oncol. 1999;17:3461–3467. - PubMed
-
- Mulders PF, Schalken JA. Measuring therapeutic efficacy in the changing paradigm of castrate-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2009;12:241–246. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
