PTEN loss increases PD-L1 protein expression and affects the correlation between PD-L1 expression and clinical parameters in colorectal cancer
- PMID: 23785454
- PMCID: PMC3681867
- DOI: 10.1371/journal.pone.0065821
PTEN loss increases PD-L1 protein expression and affects the correlation between PD-L1 expression and clinical parameters in colorectal cancer
Abstract
Background: Programmed death ligand-1 (PD-L1) has been identified as a factor associated with poor prognosis in a range of cancers, and was reported to be mainly induced by PTEN loss in gliomas. However, the clinical effect of PD-L1 and its regulation by PTEN has not yet been determined in colorectal cancer (CRC). In the present study, we verified the regulation of PTEN on PD-L1 and further determined the effect of PTEN on the correlation between PD-L1 expression and clinical parameters in CRC.
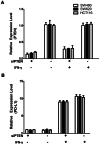
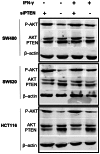
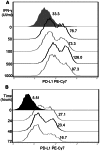
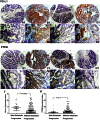
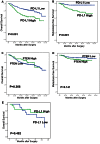
Methods/results: RNA interference approach was used to down-regulate PTEN expression in SW480, SW620 and HCT116 cells. It was showed that PD-L1 protein, but not mRNA, was significantly increased in cells transfected with siRNA PTEN compared with the negative control. Moreover, the capacity of PTEN to regulate PD-L1 expression was not obviously affected by IFN-γ, the main inducer of PD-L1. Tissue microarray immunohistochemistry was used to detect PD-L1 and PTEN in 404 CRC patient samples. Overexpression of PD-L1 was significantly correlated with distant metastasis (P<0.001), TNM stage (P<0.01), metastatic progression (P<0.01) and PTEN expression (P<0.001). Univariate analysis revealed that patients with high PD-L1 expression had a poor overall survival (P<0.001). However, multivariate analysis did not support PD-L1 as an independent prognostic factor (P = 0.548). Univariate (P<0.001) and multivariate survival (P<0.001) analysis of 310 located CRC patients revealed that high level of PD-L1 expression was associated with increased risks of metastatic progression. Furthermore, the clinical effect of PD-L1 on CRC was not statistically significant in a subset of 39 patients with no PTEN expression (distant metastasis: P = 0.102; TNM stage: P = 0.634, overall survival: P = 0.482).
Conclusions: PD-L1 can be used to identify CRC patients with high risk of metastasis and poor prognosis. This clinical manifestation may be partly associated with PTEN expression.
Conflict of interest statement
Figures

References
-
- Jemal A, Bray F (2011) Center MM, Ferlay J, Ward E, et al (2011) Global cancer statistics. CA Cancer J Clin 61: 69–90. - PubMed
-
- Bretthauer M (2010) Evidence for colorectal cancer screening. Best Pract Res Clin Gastroenterol 24: 417–425. - PubMed
-
- Zou W, Chen L (2008) Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 8: 467–477. - PubMed
-
- Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, et al. (2002) Expression of programmed death 1 ligands by murine T cells and APC. J Immunol 169: 5538–5545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

