Quantum and all-atom molecular dynamics simulations of protonation and divalent ion binding to phosphatidylinositol 4,5-bisphosphate (PIP2)
- PMID: 23786273
- PMCID: PMC3930568
- DOI: 10.1021/jp401414y
Quantum and all-atom molecular dynamics simulations of protonation and divalent ion binding to phosphatidylinositol 4,5-bisphosphate (PIP2)
Abstract
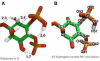
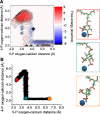
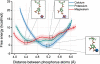
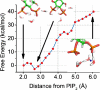
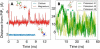
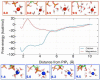
Molecular dynamics calculations have been used to determine the structure of phosphatidylinositol 4,5 bisphosphate (PIP2) at the quantum level and to quantify the propensity for PIP2 to bind two physiologically relevant divalent cations, Mg(2+) and Ca(2+). We performed a geometry optimization at the Hartree-Fock 6-31+G(d) level of theory in vacuum and with a polarized continuum dielectric to determine the conformation of the phospholipid headgroup in the presence of water and its partial charge distribution. The angle between the headgroup and the acyl chains is nearly perpendicular, suggesting that in the absence of other interactions the inositol ring would lie flat along the cytoplasmic surface of the plasma membrane. Next, we employed hybrid quantum mechanics/molecular mechanics (QM/MM) simulations to investigate the protonation state of PIP2 and its interactions with magnesium or calcium. We test the hypothesis suggested by prior experiments that binding of magnesium to PIP2 is mediated by a water molecule that is absent when calcium binds. These results may explain the selective ability of calcium to induce the formation of PIP2 clusters and phase separation from other lipids.
Figures
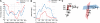
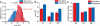
Similar articles
-
Metal ions and phosphatidylinositol 4,5-bisphosphate as interacting effectors of α-type plant phospholipase D.Phytochemistry. 2017 Jun;138:57-64. doi: 10.1016/j.phytochem.2017.02.024. Epub 2017 Mar 7. Phytochemistry. 2017. PMID: 28283189
-
The structure of divalent cation-induced aggregates of PIP2 and their alteration by gelsolin and tau.Biophys J. 1997 Sep;73(3):1440-7. doi: 10.1016/S0006-3495(97)78176-1. Biophys J. 1997. PMID: 9284311 Free PMC article.
-
Ion-Induced PIP2 Clustering with Martini3: Modification of Phosphate-Ion Interactions and Comparison with CHARMM36.J Phys Chem B. 2024 Mar 7;128(9):2134-2143. doi: 10.1021/acs.jpcb.3c06523. Epub 2024 Feb 23. J Phys Chem B. 2024. PMID: 38393820 Free PMC article.
-
PIP2Clustering: From model membranes to cells.Chem Phys Lipids. 2015 Nov;192:33-40. doi: 10.1016/j.chemphyslip.2015.07.021. Epub 2015 Jul 29. Chem Phys Lipids. 2015. PMID: 26232664 Review.
-
PIP2 is a necessary cofactor for ion channel function: how and why?Annu Rev Biophys. 2008;37:175-95. doi: 10.1146/annurev.biophys.37.032807.125859. Annu Rev Biophys. 2008. PMID: 18573078 Free PMC article. Review.
Cited by
-
Structural basis for interdomain communication in SHIP2 providing high phosphatase activity.Elife. 2017 Aug 9;6:e26640. doi: 10.7554/eLife.26640. Elife. 2017. PMID: 28792888 Free PMC article.
-
Characterization of the membrane interactions of phospholipase Cγ reveals key features of the active enzyme.Sci Adv. 2022 Jun 24;8(25):eabp9688. doi: 10.1126/sciadv.abp9688. Epub 2022 Jun 24. Sci Adv. 2022. PMID: 35749497 Free PMC article.
-
The Influence of Phosphoinositide Lipids in the Molecular Biology of Membrane Proteins: Recent Insights from Simulations.J Mol Biol. 2025 Feb 15;437(4):168937. doi: 10.1016/j.jmb.2025.168937. Epub 2025 Jan 9. J Mol Biol. 2025. PMID: 39793883 Free PMC article. Review.
-
Counterion-mediated cluster formation by polyphosphoinositides.Chem Phys Lipids. 2014 Sep;182:38-51. doi: 10.1016/j.chemphyslip.2014.01.001. Epub 2014 Jan 15. Chem Phys Lipids. 2014. PMID: 24440472 Free PMC article. Review.
-
Calcium Directly Regulates Phosphatidylinositol 4,5-Bisphosphate Headgroup Conformation and Recognition.J Am Chem Soc. 2017 Mar 22;139(11):4019-4024. doi: 10.1021/jacs.6b11760. Epub 2017 Mar 7. J Am Chem Soc. 2017. PMID: 28177616 Free PMC article.
References
-
- Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular Function of Phosphoinositide 3-Kinases: Implications for Development, Homeostasis, and Cancer. Annu. Rev. Cell Dev. Biol. 2001;17:615–675. - PubMed
-
- Lassing I, Lindberg U. Polyphosphoinositide Synthesis in Platelets Stimulated with Low Concentrations of Thrombin Is Enhanced Before the Activation of Phospholipase C. FEBS Lett. 1990;262:231–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

