Aicardi-Goutières syndrome: a model disease for systemic autoimmunity
- PMID: 23786362
- PMCID: PMC3898550
- DOI: 10.1111/cei.12160
Aicardi-Goutières syndrome: a model disease for systemic autoimmunity
Abstract
Systemic autoimmunity is a complex disease process that results from a loss of immunological tolerance characterized by the inability of the immune system to discriminate self from non-self. In patients with the prototypic autoimmune disease systemic lupus erythematosus (SLE), formation of autoantibodies targeting ubiquitous nuclear antigens and subsequent deposition of immune complexes in the vascular bed induces inflammatory tissue injury that can affect virtually any organ system. Given the extraordinary genetic and phenotypic heterogeneity of SLE, one approach to the genetic dissection of complex SLE is to study monogenic diseases, for which a single gene defect is responsible. Considerable success has been achieved from the analysis of the rare monogenic disorder Aicardi-Goutières syndrome (AGS), an inflammatory encephalopathy that clinically resembles in-utero-acquired viral infection and that also shares features with SLE. Progress in understanding the cellular and molecular functions of the AGS causing genes has revealed novel pathways of the metabolism of intracellular nucleic acids, the major targets of the autoimmune attack in patients with SLE. Induction of autoimmunity initiated by immune recognition of endogenous nucleic acids originating from processes such as DNA replication/repair or endogenous retro-elements represents novel paradigms of SLE pathogenesis. These findings illustrate how investigating rare monogenic diseases can also fuel discoveries that advance our understanding of complex disease. This will not only aid the development of improved tools for SLE diagnosis and disease classification, but also the development of novel targeted therapeutic approaches.
Keywords: Aicardi-Goutières syndrome; autoimmunity; nucleic acid sensing; systemic lupus erythematosus; type I interferon.
© 2013 British Society for Immunology.
Figures
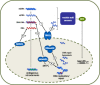
References
-
- Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–939. - PubMed
-
- Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. - PubMed
-
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. - PubMed
-
- Alarcon GS, McGwin G, Jr, Roseman JM, et al. Systemic lupus erythematosus in three ethnic groups. XIX. Natural history of the accrual of the American College of Rheumatology criteria prior to the occurrence of criteria diagnosis. Arthritis Rheum. 2004;51:609–615. - PubMed
-
- Danchenko N, Satia JA, Anthony MS. Epidemiology of systemic lupus erythematosus: a comparison of worldwide disease burden. Lupus. 2006;15:308–318. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

