The expression of MC4Rs in D1R neurons regulates food intake and locomotor sensitization to cocaine
- PMID: 23786641
- PMCID: PMC3782389
- DOI: 10.1111/gbb.12057
The expression of MC4Rs in D1R neurons regulates food intake and locomotor sensitization to cocaine
Abstract
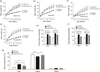
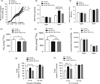
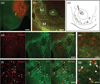
While it is known that mice lacking melanocortin 4 receptor (MC4R) expression develop hyperphagia resulting in early-onset obesity, the specific neural circuits that mediate this process remain unclear. Here, we report that selective restoration of MC4R expression within dopamine-1 receptor-expressing neurons [MC4R/dopamine 1 receptor (D1R) mice] partially blunts the severe obesity seen in MC4R-null mice by decreasing meal size, but not meal frequency, in the dark cycle. We also report that both acute cocaine-induced anorexia and the development of locomotor sensitization to repeated administration of cocaine are blunted in MC4R-null mice and normalized in MC4R/D1R mice. Neuronal retrograde tracing identifies the lateral hypothalamic area as the primary target of MC4R-expressing neurons in the nucleus accumbens. Biochemical studies in the ventral striatum show that phosphorylation of DARPP-32(Thr) (-34) and GluR1(Ser) (-845) is diminished in MC4R-null mice after chronic cocaine administration but rescued in MC4R/D1R mice. These findings highlight a physiological role of MC4R-mediated signaling within D1R neurons in the long-term regulation of energy balance and behavioral responses to cocaine.
Keywords: Cocaine; dopamine-1 receptor; food intake; locomotor sensitization; melanocortin 4 receptor; obesity.
© 2013 John Wiley & Sons Ltd and International Behavioural and Neural Genetics Society.
Figures

Similar articles
-
Melanocortin 4 receptor signaling in dopamine 1 receptor neurons is required for procedural memory learning.Physiol Behav. 2012 May 15;106(2):201-10. doi: 10.1016/j.physbeh.2012.01.025. Epub 2012 Feb 9. Physiol Behav. 2012. PMID: 22342812 Free PMC article.
-
Cocaine-Dependent Acquisition of Locomotor Sensitization and Conditioned Place Preference Requires D1 Dopaminergic Signaling through a Cyclic AMP, NCS-Rapgef2, ERK, and Egr-1/Zif268 Pathway.J Neurosci. 2021 Jan 27;41(4):711-725. doi: 10.1523/JNEUROSCI.1497-20.2020. Epub 2020 Dec 2. J Neurosci. 2021. PMID: 33268547 Free PMC article.
-
Dynamic dichotomy of accumbal population activity underlies cocaine sensitization.Elife. 2021 Oct 5;10:e66048. doi: 10.7554/eLife.66048. Elife. 2021. PMID: 34608866 Free PMC article.
-
Peripheral actions and direct central-local communications of melanocortin 4 receptor signaling.J Sport Health Sci. 2023 Jan;12(1):45-51. doi: 10.1016/j.jshs.2021.02.001. Epub 2021 Feb 20. J Sport Health Sci. 2023. PMID: 33621697 Free PMC article. Review.
-
Defining Hyperphagia for Improved Diagnosis and Management of MC4R Pathway-Associated Disease: A Roundtable Summary.Curr Obes Rep. 2025 Jan 25;14(1):13. doi: 10.1007/s13679-024-00601-z. Curr Obes Rep. 2025. PMID: 39856371 Free PMC article. Review.
Cited by
-
MC4R expression in pedunculopontine nucleus involved in the modulation of midbrain dopamine system.Int J Clin Exp Pathol. 2015 Feb 1;8(2):2039-43. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 25973101 Free PMC article.
-
Behavioral Alterations in Mice Carrying Homozygous HDAC4 A778T Missense Mutation Associated With Eating Disorder.Front Neurosci. 2020 Feb 21;14:139. doi: 10.3389/fnins.2020.00139. eCollection 2020. Front Neurosci. 2020. PMID: 32153359 Free PMC article.
-
The Role of the Melanocortin System in Metabolic Disease: New Developments and Advances.Neuroendocrinology. 2017;104(4):330-346. doi: 10.1159/000450649. Epub 2016 Oct 11. Neuroendocrinology. 2017. PMID: 27728914 Free PMC article. Review.
-
Cognitive and autonomic determinants of energy homeostasis in obesity.Nat Rev Endocrinol. 2015 Aug;11(8):489-501. doi: 10.1038/nrendo.2015.103. Epub 2015 Jun 30. Nat Rev Endocrinol. 2015. PMID: 26122319 Review.
-
Cocaine decreases saccharin preference without altering sweet taste sensitivity.Pharmacol Biochem Behav. 2015 Jun;133:18-24. doi: 10.1016/j.pbb.2015.03.010. Epub 2015 Mar 24. Pharmacol Biochem Behav. 2015. PMID: 25812471 Free PMC article.
References
-
- Albarado DC, McClaine J, Stephens JM, Mynatt RL, Ye J, Bannon AW, Richards WG, Butler AA. Impaired coordination of nutrient intake and substrate oxidation in melanocortin-4 receptor knockout mice. Endocrinology. 2004;145:243–252. - PubMed
-
- Alvaro JD, Taylor JR, Duman RS. Molecular and behavioral interactions between central melanocortins and cocaine. J Pharmacol Exp Ther. 2003;304:391–399. - PubMed
-
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. - PubMed
-
- Boghossian S, Park M, York DA. Melanocortin activity in the amygdala controls appetite for dietary fat. Am J Physiol Regul Integr Comp Physiol. 2010;298:R385–R393. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

