Development of highly selective casein kinase 1δ/1ε (CK1δ/ε) inhibitors with potent antiproliferative properties
- PMID: 23787102
- PMCID: PMC3783656
- DOI: 10.1016/j.bmcl.2013.05.075
Development of highly selective casein kinase 1δ/1ε (CK1δ/ε) inhibitors with potent antiproliferative properties
Abstract
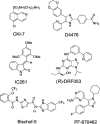
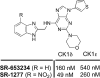
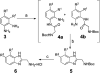
The development of a series of potent and highly selective casein kinase 1δ/ε (CK1δ/ε) inhibitors is described. Starting from a purine scaffold inhibitor (SR-653234) identified by high throughput screening, we developed a series of potent and highly kinase selective inhibitors, including SR-2890 and SR-3029, which have IC₅₀ ≤ 50 nM versus CK1δ. The two lead compounds have ≤100 nM EC50 values in MTT assays against the human A375 melanoma cell line and have physical, in vitro and in vivo PK properties suitable for use in proof of principle animal xenograft studies against human cancer cell lines.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
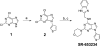

References
-
- Cheong JK, Virshup DM. Int J Biochem Cell Biol. 2011;43:465. - PubMed
-
- Price MA. Genes Dev. 2006;20:399. - PubMed
-
- Peters JM, McKay RM, McKay JP, Graff JM. Nature. 1999;401:345. - PubMed
-
- Pooler AM, Usardi A, Evans CJ, Philpott KL, Noble W, Hanger DP. Neurobiol Aging. 2012;22:431 e27. - PubMed
-
- Li G, Yin H, Kuret J. J Biol Chem. 2004;279:15938. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

