The effect of lipid monolayer in-plane rigidity on in vivo microbubble circulation persistence
- PMID: 23787108
- PMCID: PMC3762333
- DOI: 10.1016/j.biomaterials.2013.05.053
The effect of lipid monolayer in-plane rigidity on in vivo microbubble circulation persistence
Abstract
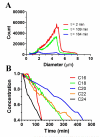
The goal of this study was to increase in vivo microbubble circulation persistence for applications in medical imaging and targeted drug delivery. Our approach was to investigate the effect of lipid monolayer in-plane rigidity to reduce the rate of microbubble dissolution, while holding constant the microbubble size, concentration and surface architecture. We first estimated the impact of acyl chain length of the main diacyl phosphatidylcholine (PC) lipid and inter-lipid distance on the cohesive surface energy and, based on these results, we hypothesized that microbubble stability and in vivo ultrasound contrast persistence would increase monotonically with increasing acyl chain length. We therefore measured microbubble in vitro stability to dilution with and without ultrasound exposure, as well as in vivo ultrasound contrast persistence. All measurements showed a sharp rise in stability between DPPC (C16:0) and DSPC (C18:0), which correlates to the wrinkling transition, signaling the onset of significant surface shear and gas permeation resistance, observed in prior single-bubble dissolution studies. Further evidence for the effect of the wrinkling transition came from an in vitro and in vivo stability comparison of microbubbles coated with pure DPPC with those of lung surfactant extract. Microbubble stability against dilution without ultrasound and in vivo ultrasound contrast persistence showed a monotonic increase with acyl chain length from DSPC to DBPC (C22:0). However, we also observed that stability dropped precipitously for all measurements on further increasing lipid acyl chain length from DBPC to DLiPC (C24:0). This result suggests that hydrophobic mismatch between the main PC lipid and the lipopolymer emulsifier, DSPE-PEG5000, may drive a less stable surface microstructure. Overall, these results support our general hypothesis of the role of in-plane rigidity for increasing the lifetime of microbubble circulation.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures






References
-
- Lindner JR. Microbubbles in medical imaging: current applications and future directions. Nat Rev Drug Discov. 2004;3:527–32. - PubMed
-
- Klibanov AL, Hughes MS, Wojdyla JK, Wible JH, Brandenburger GH. Destruction of contrast agent microbubbles in the ultrasound field: the fate of the microbubble shell and the importance of the bubble gas content. Acad Radiol. 2002;9:S41–S5. - PubMed
-
- Unger EC, Porter T, Culp W, Labell R, Matsunaga T, Zutshi R. Therapeutic applications of lipid-coated microbubbles. Adv Drug Deliv Rev. 2004;56:1291–314. - PubMed
-
- Kwan JJ, Borden MA. Lipid monolayer dilatational mechanics during microbubble gas exchange. Soft Matter. 2012;8:4756–66.
-
- Stride E. Physical principles of microbubbles for ultrasound imaging and therapy. Cerebrovasc Dis. 2009;27(Suppl 2):1–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

