Stereoretentive Pd-catalysed Stille cross-coupling reactions of secondary alkyl azastannatranes and aryl halides
- PMID: 23787752
- PMCID: PMC8048766
- DOI: 10.1038/nchem.1652
Stereoretentive Pd-catalysed Stille cross-coupling reactions of secondary alkyl azastannatranes and aryl halides
Abstract
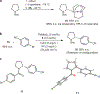
The development of transition metal-catalysed cross-coupling reactions has greatly influenced the manner in which the synthesis of complex organic molecules is approached. A wide variety of methods are now available for the formation of C(sp(2))-C(sp(2)) bonds, and more recent work has focused on the use of C(sp(3)) electrophiles and nucleophiles. The use of secondary and tertiary alkyl nucleophiles in cross-coupling reactions remains a challenge because of the propensity of these alkyl groups to isomerize under the reaction conditions. Here, we report the development of a general Pd-catalysed process for the stereoretentive cross-coupling of secondary alkyl azastannatrane nucleophiles with aryl chlorides, bromides, iodides and triflates. Coupling partners with a wide range of electronic characteristics are well tolerated. The reaction occurs with minimal isomerization of the secondary alkyltin nucleophile, and with retention of absolute configuration. This process constitutes the first general method to use secondary alkyltin reagents in cross-coupling reactions.
Conflict of interest statement
Competing financial interests
The authors declare no competing financial interests.
Figures

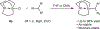
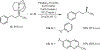
References
-
- De Meijere A & Diederich F (eds) Metal-Catalysed Cross-Coupling Reactions (Wiley-VCH, 2004).
-
- Netherton MR & Fu GC in Topics in Organometallic Chemistry: Palladium in Organic Synthesis (ed. Tsuji J) 85–108 (Springer, 2005).
-
- Rudolph A & Lautens M Secondary alkyl halides in transition metal-catalyzed cross-coupling reactions. Angew. Chem. Int. Ed 48, 2656–2670 (2009). - PubMed
-
- Chemler SR, Trauner D & Danishefsky SJ The β-alkyl Suzuki–Miyaura cross-coupling reaction: development, mechanistic study, and applications in natural product synthesis. Angew. Chem. Int. Ed 40, 4544–4568 (2001). - PubMed
-
- Luo X et al. Superior effect of a π-acceptor ligand (phosphine-electron-deficient olefin ligand) in the Negishi coupling involving alkylzinc reagents. Org. Lett 9, 4571–4574 (2007). - PubMed
Publication types
MeSH terms
Substances
Associated data
- PubChem-Substance/162163905
- PubChem-Substance/162163906
- PubChem-Substance/162163907
- PubChem-Substance/162163908
- PubChem-Substance/162163909
- PubChem-Substance/162163910
- PubChem-Substance/162163911
- PubChem-Substance/162163912
- PubChem-Substance/162163913
- PubChem-Substance/162163914
- PubChem-Substance/162163915
- PubChem-Substance/162163916
- PubChem-Substance/162163917
- PubChem-Substance/162163918
- PubChem-Substance/162163919
- PubChem-Substance/162163920
- PubChem-Substance/162163921
- PubChem-Substance/162163922
- PubChem-Substance/162163923
- PubChem-Substance/162163924
- PubChem-Substance/162163925
- PubChem-Substance/162163926
- PubChem-Substance/162163927
- PubChem-Substance/162163928
- PubChem-Substance/162163929
- PubChem-Substance/162163930
- PubChem-Substance/162163931
- PubChem-Substance/162163932
- PubChem-Substance/162163933
- PubChem-Substance/162163934
- PubChem-Substance/162163935
- PubChem-Substance/162163936
- PubChem-Substance/162163937
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

