Blebs produced by actin-myosin contraction during apoptosis release damage-associated molecular pattern proteins before secondary necrosis occurs
- PMID: 23787996
- PMCID: PMC3770329
- DOI: 10.1038/cdd.2013.69
Blebs produced by actin-myosin contraction during apoptosis release damage-associated molecular pattern proteins before secondary necrosis occurs
Abstract
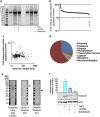
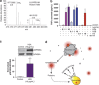
Apoptosis is a fundamental homeostatic mechanism essential for the normal growth, development and maintenance of every tissue and organ. Dying cells have been defined as apoptotic by distinguishing features, including cell contraction, nuclear fragmentation, blebbing, apoptotic body formation and maintenance of intact cellular membranes to prevent massive protein release and consequent inflammation. We now show that during early apoptosis limited membrane permeabilization occurs in blebs and apoptotic bodies, which allows release of proteins that may affect the proximal microenvironment before the catastrophic loss of membrane integrity during secondary necrosis. Blebbing, apoptotic body formation and protein release during early apoptosis are dependent on ROCK and myosin ATPase activity to drive actomyosin contraction. We identified 231 proteins released from actomyosin contraction-dependent blebs and apoptotic bodies by adapted SILAC (stable isotope labeling with amino acids in cell culture) combined with mass spectrometry analysis. The most enriched proteins released were the nucleosomal histones, which have previously been identified as damage-associated molecular pattern proteins (DAMPs) that can initiate sterile inflammatory responses. These results indicate that limited membrane permeabilization occurs in blebs and apoptotic bodies before secondary necrosis, leading to acute and localized release of immunomodulatory proteins during the early phase of active apoptotic membrane blebbing. Therefore, the shift from apoptosis to secondary necrosis is more graded than a simple binary switch, with the membrane permeabilization of apoptotic bodies and consequent limited release of DAMPs contributing to the transition between these states.
Figures





References
-
- Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–788. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

