Immunogenic calreticulin exposure occurs through a phylogenetically conserved stress pathway involving the chemokine CXCL8
- PMID: 23787997
- PMCID: PMC3857625
- DOI: 10.1038/cdd.2013.73
Immunogenic calreticulin exposure occurs through a phylogenetically conserved stress pathway involving the chemokine CXCL8
Abstract
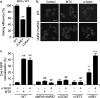
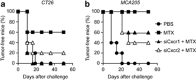
The exposure of calreticulin (CRT) on the surface of stressed and dying cancer cells facilitates their uptake by dendritic cells and the subsequent presentation of tumor-associated antigens to T lymphocytes, hence stimulating an anticancer immune response. The chemotherapeutic agent mitoxantrone (MTX) can stimulate the peripheral relocation of CRT in both human and yeast cells, suggesting that the CRT exposure pathway is phylogenetically conserved. Here, we show that pheromones can act as physiological inducers of CRT exposure in yeast cells, thereby facilitating the formation of mating conjugates, and that a large-spectrum inhibitor of G protein-coupled receptors (which resemble the yeast pheromone receptor) prevents CRT exposure in human cancer cells exposed to MTX. An RNA interference screen as well as transcriptome analyses revealed that chemokines, in particular human CXCL8 (best known as interleukin-8) and its mouse ortholog Cxcl2, are involved in the immunogenic translocation of CRT to the outer leaflet of the plasma membrane. MTX stimulated the production of CXCL8 by human cancer cells in vitro and that of Cxcl2 by murine tumors in vivo. The knockdown of CXCL8/Cxcl2 receptors (CXCR1/Cxcr1 and Cxcr2) reduced MTX-induced CRT exposure in both human and murine cancer cells, as well as the capacity of the latter-on exposure to MTX-to elicit an anticancer immune response in vivo. Conversely, the addition of exogenous Cxcl2 increased the immunogenicity of dying cells in a CRT-dependent manner. Altogether, these results identify autocrine and paracrine chemokine signaling circuitries that modulate CRT exposure and the immunogenicity of cell death.
Figures




References
-
- Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–1577. - PubMed
-
- Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

