Entecavir and hepatitis B immune globulin in patients undergoing liver transplantation for chronic hepatitis B
- PMID: 23788462
- PMCID: PMC3791551
- DOI: 10.1002/lt.23690
Entecavir and hepatitis B immune globulin in patients undergoing liver transplantation for chronic hepatitis B
Abstract
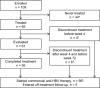
For patients undergoing liver transplantation (LT) for hepatitis B virus (HBV)-related liver disease, the current standard of care for preventing reinfection of the allograft is nucleoside analogue therapy combined with hepatitis B immune globulin (HBIG). Entecavir has demonstrated high efficacy and a favorable safety profile for chronic hepatitis B (CHB) treatment, but data for patients undergoing HBV-related LT are limited. This study assessed the safety and efficacy of entecavir combined with various HBIG regimens after CHB-related LT. In this phase 3b, single-arm, open-label study, 65 patients undergoing LT for CHB-related liver disease with an HBV DNA load <172 IU/mL at LT received entecavir (1.0 mg daily) for 72 weeks after LT. The primary endpoint was the proportion of evaluable patients (treated for ≥4 weeks) with virological recurrence (HBV DNA level ≥50 IU/mL) through week 72. Concomitant HBIG therapy was received by 64 of the 65 enrolled patients, and 44% of these patients received high-dose HBIG (any HBIG dose in the specified interval ≥10,000 IU). Through week 72, all 61 patients evaluable for the efficacy analysis had undetectable HBV DNA. The Kaplan-Meier estimate of patients without hepatitis B surface antigen (HBsAg) recurrence at week 72 was 0.9655. Two patients experienced a reappearance of HBsAg, but both remained HBV DNA(-) until the last follow-up. The frequency and nature of adverse events were consistent with those expected for this patient population. Serum creatinine increments ≥0.3 mg/dL and ≥0.5 mg/dL occurred in 62% and 39% of the patients, respectively, and all of these patients received calcineurin inhibitor therapy. In conclusion, in this population of patients treated with entecavir after CHB-related LT, entecavir was well tolerated and effective in maintaining viral suppression, even in individuals who experienced a reappearance of HBsAg.
© 2013 American Association for the Study of Liver Diseases.
Figures

Similar articles
-
Section 14. Combination of entecavir plus low-dose on-demand hepatitis B immunoglobulin is effective with very low hepatitis B recurrence after liver transplantation.Transplantation. 2014 Apr 27;97 Suppl 8:S53-9. doi: 10.1097/01.tp.0000446278.43804.f9. Transplantation. 2014. PMID: 24849836
-
Living related liver transplantation for hepatitis B-related liver disease without hepatitis B immune globulin prophylaxis.Liver Transpl. 2013 Sep;19(9):1030-5. doi: 10.1002/lt.23692. Epub 2013 Aug 18. Liver Transpl. 2013. PMID: 23788470
-
Entecavir Monotherapy Prevents Hepatitis B Virus Recurrence After Liver Transplant for Chronic Hepatitis B Patients: A Long-Term Retrospective Study.Transplant Proc. 2021 Jun;53(5):1700-1706. doi: 10.1016/j.transproceed.2021.04.007. Epub 2021 May 22. Transplant Proc. 2021. PMID: 34030872
-
High genetic barrier nucleos(t)ide analogue(s) for prophylaxis from hepatitis B virus recurrence after liver transplantation: a systematic review.Am J Transplant. 2013 Feb;13(2):353-62. doi: 10.1111/j.1600-6143.2012.04315.x. Epub 2012 Nov 8. Am J Transplant. 2013. PMID: 23137006
-
Transplantation of hepatitis D virus patients: Lifelong hepatitis B immunoglobulins?Liver Int. 2023 Aug;43 Suppl 1:96-100. doi: 10.1111/liv.15352. Epub 2022 Jul 9. Liver Int. 2023. PMID: 35767373 Review.
Cited by
-
Prophylactic managements of hepatitis B viral infection in liver transplantation.World J Gastroenterol. 2016 Jan 7;22(1):165-75. doi: 10.3748/wjg.v22.i1.165. World J Gastroenterol. 2016. PMID: 26755868 Free PMC article. Review.
-
Contradictory immune response in post liver transplantation hepatitis B and C.Int J Inflam. 2014;2014:814760. doi: 10.1155/2014/814760. Epub 2014 Aug 24. Int J Inflam. 2014. PMID: 25215259 Free PMC article. Review.
-
Safe and cost-effective control of post-transplantation recurrence of hepatitis B.Hepatol Res. 2015 Jan;45(1):38-47. doi: 10.1111/hepr.12368. Epub 2014 Jul 18. Hepatol Res. 2015. PMID: 24905970 Free PMC article.
-
Recent advances in prevention of hepatitis B recurrence after liver transplantation.World J Gastroenterol. 2015 Jan 21;21(3):829-35. doi: 10.3748/wjg.v21.i3.829. World J Gastroenterol. 2015. PMID: 25624716 Free PMC article. Review.
-
Rational Basis for Optimizing Short and Long-term Hepatitis B Virus Prophylaxis Post Liver Transplantation: Role of Hepatitis B Immune Globulin.Transplantation. 2015 Jul;99(7):1321-34. doi: 10.1097/TP.0000000000000777. Transplantation. 2015. PMID: 26038873 Free PMC article. Review.
References
-
- Maddrey WC. Hepatitis B: an important public health issue. J Med Virol. 2000;61:362–366. - PubMed
-
- Terrault N, Roche B, Samuel D. Management of the hepatitis B virus in the liver transplantation setting: a European and an American perspective. Liver Transpl. 2005;11:716–732. - PubMed
-
- Katz LH, Paul M, Guy DG, Tur-Kaspa R. Prevention of recurrent hepatitis B virus infection after liver transplantation: hepatitis B immunoglobulin, antiviral drugs, or both? Systematic review and meta-analysis. Transpl Infect Dis. 2010;12:292–308. - PubMed
-
- McCaughan GW. Prevention of post liver transplant HBV recurrence. Hepatol Int. 2011;5:876–881. - PubMed
-
- Perrillo RP, Wright T, Rakela J, Levy G, Schiff E, Gish R, et al. for Lamivudine North American Transplant Group A multicenter United States-Canadian Trial to assess lamivudine monotherapy before and after liver transplantation for chronic hepatitis B. Hepatology. 2001;33:424–432. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
