Deletion of MCL-1 causes lethal cardiac failure and mitochondrial dysfunction
- PMID: 23788622
- PMCID: PMC3701191
- DOI: 10.1101/gad.215855.113
Deletion of MCL-1 causes lethal cardiac failure and mitochondrial dysfunction
Abstract
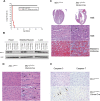
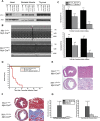
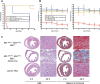
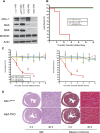
MCL-1 is an essential BCL-2 family member that promotes the survival of multiple cellular lineages, but its role in cardiac muscle has remained unclear. Here, we report that cardiac-specific ablation of Mcl-1 results in a rapidly fatal, dilated cardiomyopathy manifested by a loss of cardiac contractility, abnormal mitochondria ultrastructure, and defective mitochondrial respiration. Strikingly, genetic ablation of both proapoptotic effectors (Bax and Bak) could largely rescue the lethality and impaired cardiac function induced by Mcl-1 deletion. However, while the overt consequences of Mcl-1 loss were obviated by combining with the loss of Bax and Bak, mitochondria from the Mcl-1-, Bax-, and Bak-deficient hearts still revealed mitochondrial ultrastructural abnormalities and displayed deficient mitochondrial respiration. Together, these data indicate that merely blocking cell death is insufficient to completely overcome the need for MCL-1 function in cardiomyocytes and suggest that in cardiac muscle, MCL-1 also facilitates normal mitochondrial function. These findings are important, as specific MCL-1-inhibiting therapeutics are being proposed to treat cancer cells and may result in unexpected cardiac toxicity.
Keywords: BCL-2; MCL-1; apoptosis; heart failure; mitochondria.
Figures

References
-
- Brocheriou V, Hagege AA, Oubenaissa A, Lambert M, Mallet VO, Duriez M, Wassef M, Kahn A, Menasche P, Gilgenkrantz H 2000. Cardiac functional improvement by a human Bcl-2 transgene in a mouse model of ischemia/reperfusion injury. J Gene Med 2: 326–333 - PubMed
-
- Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A 2006. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell 9: 351–365 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
