α4α6β2* nicotinic acetylcholine receptor activation on ventral tegmental area dopamine neurons is sufficient to stimulate a depolarizing conductance and enhance surface AMPA receptor function
- PMID: 23788655
- PMCID: PMC3876818
- DOI: 10.1124/mol.113.087346
α4α6β2* nicotinic acetylcholine receptor activation on ventral tegmental area dopamine neurons is sufficient to stimulate a depolarizing conductance and enhance surface AMPA receptor function
Abstract
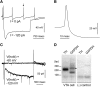
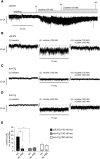
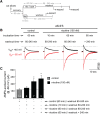
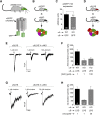
Tobacco addiction is a serious threat to public health in the United States and abroad, and development of new therapeutic approaches is a major priority. Nicotine activates and/or desensitizes nicotinic acetylcholine receptors (nAChRs) throughout the brain. nAChRs in ventral tegmental area (VTA) dopamine (DA) neurons are crucial for the rewarding and reinforcing properties of nicotine in rodents, suggesting that they may be key mediators of nicotine's action in humans. However, it is unknown which nAChR subtypes are sufficient to activate these neurons. To test the hypothesis that nAChRs containing α6 subunits are sufficient to activate VTA DA neurons, we studied mice expressing hypersensitive, gain-of-function α6 nAChRs (α6L9'S mice). In voltage-clamp recordings in brain slices from adult mice, 100 nM nicotine was sufficient to elicit inward currents in VTA DA neurons via α6β2* nAChRs. In addition, we found that low concentrations of nicotine could act selectively through α6β2* nAChRs to enhance the function of 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl)propanoic acid (AMPA) receptors on the surface of these cells. In contrast, α6β2* activation did not enhance N-methyl-D-aspartic acid receptor function. Finally, AMPA receptor (AMPAR) function was not similarly enhanced in brain slices from α6L9'S mice lacking α4 nAChR subunits, suggesting that α4α6β2* nAChRs are important for enhancing AMPAR function in VTA DA neurons. Together, these data suggest that activation of α4α6β2* nAChRs in VTA DA neurons is sufficient to support the initiation of cellular changes that play a role in addiction to nicotine. α4α6β2* nAChRs may be a promising target for future smoking cessation pharmacotherapy.
Figures




References
-
- Bellone C, Lüscher C. (2006) Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci 9:636–641 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

