Lysine acetyltransferase PCAF is a key regulator of arteriogenesis
- PMID: 23788761
- PMCID: PMC4049097
- DOI: 10.1161/ATVBAHA.113.301579
Lysine acetyltransferase PCAF is a key regulator of arteriogenesis
Abstract
Objective: Therapeutic arteriogenesis, that is, expansive remodeling of preexisting collaterals, using single-action factor therapies has not been as successful as anticipated. Modulation of factors that act as a master switch for relevant gene programs may prove more effective. Transcriptional coactivator p300-CBP-associated factor (PCAF) has histone acetylating activity and promotes transcription of multiple inflammatory genes. Because arteriogenesis is an inflammation-driven process, we hypothesized that PCAF acts as multifactorial regulator of arteriogenesis.
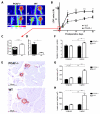
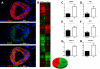
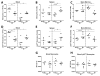
Approach and results: After induction of hindlimb ischemia, blood flow recovery was impaired in both PCAF(-/-) mice and healthy wild-type mice treated with the pharmacological PCAF inhibitor Garcinol, demonstrating an important role for PCAF in arteriogenesis. PCAF deficiency reduced the in vitro inflammatory response in leukocytes and vascular cells involved in arteriogenesis. In vivo gene expression profiling revealed that PCAF deficiency results in differential expression of 3505 genes during arteriogenesis and, more specifically, in impaired induction of multiple proinflammatory genes. Additionally, recruitment from the bone marrow of inflammatory cells, in particular proinflammatory Ly6C(hi) monocytes, was severely impaired in PCAF(-/-) mice.
Conclusions: These findings indicate that PCAF acts as master switch in the inflammatory processes required for effective arteriogenesis.
Keywords: inflammation; monocytes; p300-CBP–associated factor; peripheral arterial disease.
Figures



References
-
- van Oostrom MC, van Oostrom O, Quax PH, Verhaar MC, Hoefer IE. Insights into mechanisms behind arteriogenesis: what does the future hold? J Leukoc Biol. 2008;84:1379–91. - PubMed
-
- Heil M, Schaper W. Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis) Circ Res. 2004;95:449–58. - PubMed
-
- Schaper J, Konig R, Franz D, Schaper W. The endothelial surface of growing coronary collateral arteries. Intimal margination and diapedesis of monocytes. A combined SEM and TEM study. Virchows Arch A Pathol Anat Histol. 1976;370:193–205. - PubMed
-
- Bergmann CE, Hoefer IE, Meder B, Roth H, van Royen N, Breit SM, Jost MM, Aharinejad S, Hartmann S, Buschmann IR. Arteriogenesis depends on circulating monocytes and macrophage accumulation and is severely depressed in op/op mice. J Leukoc Biol. 2006;80:59–65. - PubMed
-
- Heil M, Ziegelhoeffer T, Pipp F, Kostin S, Martin S, Clauss M, Schaper W. Blood monocyte concentration is critical for enhancement of collateral artery growth. Am J Physiol Heart Circ Physiol. 2002;283:H2411–H2419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

