Brevenal, a brevetoxin antagonist from Karenia brevis, binds to a previously unreported site on mammalian sodium channels
- PMID: 23789024
- PMCID: PMC3684244
- DOI: 10.1016/j.hal.2013.03.001
Brevenal, a brevetoxin antagonist from Karenia brevis, binds to a previously unreported site on mammalian sodium channels
Abstract
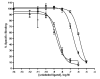
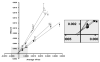
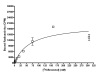
Brevetoxins are a family of ladder-frame polyether toxins produced by the marine dinoflagellate Karenia brevis. During blooms of K. brevis, inhalation of brevetoxins aerosolized by wind and wave action can lead to asthma-like symptoms in persons at the beach. Consumption of either shellfish or finfish contaminated by K. brevis blooms can lead to the development of neurotoxic shellfish poisoning. The toxic effects of brevetoxins are due to binding at a defined site on, and subsequent activation of, voltage-sensitive sodium channels (VSSCs) in cell membranes (site 5). In addition to brevetoxins, K. brevis produces several other ladder-frame compounds. One of these compounds, brevenal, has been shown to antagonize the effects of brevetoxin. In an effort to further characterize to effects of brevenal, a radioactive analog ([3H]-brevenol) was produced by reducing the side-chain terminal aldehyde moiety of brevenal to an alcohol using tritiated sodium borohydride. A KD of 67 nM and Bmax of 7.1 pmol/mg protein were obtained for [3H]-brevenol in rat brain synaptosomes, suggesting a 1:1 matching with VSSCs. Brevenal and brevenol competed for [3H]-brevenol binding with Ki values of 75 nM and 56 nM, respectively. However, although both brevenal and brevenol can inhibit brevetoxin binding, brevetoxin was completely ineffective at competition for [3H]-brevenol binding. After examining other site-specific compounds, it was determined that [3H]-brevenol binds to a site that is distinct from the other known sites including the brevetoxin site (site 5) although some interaction with site 5 is apparent.
Keywords: Brevenal, Brevetoxin; Competition Binding Assay; Radioligand Assay.
Figures

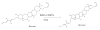


Similar articles
-
Development of a competitive fluorescence-based synaptosome binding assay for brevetoxins.Harmful Algae. 2012 Sep;19:85-91. doi: 10.1016/j.hal.2012.06.003. Harmful Algae. 2012. PMID: 22984362 Free PMC article.
-
Development of a fluorescence assay for the characterization of brevenal binding to rat brain synaptosomes.J Nat Prod. 2014 Sep 26;77(9):2014-20. doi: 10.1021/np500118p. Epub 2014 Sep 17. J Nat Prod. 2014. PMID: 25226846 Free PMC article.
-
Development and utilization of a fluorescence-based receptor-binding assay for the site 5 voltage-sensitive sodium channel ligands brevetoxin and ciguatoxin.J AOAC Int. 2014 Mar-Apr;97(2):307-15. doi: 10.5740/jaoacint.sgemccall. J AOAC Int. 2014. PMID: 24830141
-
Role of Biomarkers in Monitoring Brevetoxins in Karenia brevis Exposed Shellfish.Food Saf (Tokyo). 2018 Mar 30;6(1):33-43. doi: 10.14252/foodsafetyfscj.2017021. eCollection 2018 Mar. Food Saf (Tokyo). 2018. PMID: 32231945 Free PMC article. Review.
-
Ladder-Shaped Ion Channel Ligands: Current State of Knowledge.Mar Drugs. 2017 Jul 20;15(7):232. doi: 10.3390/md15070232. Mar Drugs. 2017. PMID: 28726749 Free PMC article. Review.
Cited by
-
Pharmacological Modulation of Ion Channels for the Treatment of Cystic Fibrosis.J Exp Pharmacol. 2021 Jul 23;13:693-723. doi: 10.2147/JEP.S255377. eCollection 2021. J Exp Pharmacol. 2021. PMID: 34326672 Free PMC article. Review.
-
Marine Toxins Detection by Biosensors Based on Aptamers.Toxins (Basel). 2019 Dec 18;12(1):1. doi: 10.3390/toxins12010001. Toxins (Basel). 2019. PMID: 31861315 Free PMC article. Review.
-
Neurological Disturbances of Ciguatera Poisoning: Clinical Features and Pathophysiological Basis.Cells. 2020 Oct 14;9(10):2291. doi: 10.3390/cells9102291. Cells. 2020. PMID: 33066435 Free PMC article. Review.
-
Structure activity relationship of brevenal hydrazide derivatives.Mar Drugs. 2014 Mar 28;12(4):1839-58. doi: 10.3390/md12041839. Mar Drugs. 2014. PMID: 24686558 Free PMC article.
-
Intraspecific Variability in the Toxin Production and Toxin Profiles of In Vitro Cultures of Gambierdiscus polynesiensis (Dinophyceae) from French Polynesia.Toxins (Basel). 2019 Dec 17;11(12):735. doi: 10.3390/toxins11120735. Toxins (Basel). 2019. PMID: 31861242 Free PMC article.
References
-
- Backer CB, Kirkpatrick B, Fleming LA, Cheung YS, Pierce R, Bean JA, Clark R, Johnson D, Wanner A, Tamer R, Zhou Y, Baden DG. Occupational exposure to aerosolized brevetoxins during Florida red tide events: Effects on a healthy worker population. Environ. Health Perspect. 2005;113:644–649. - PMC - PubMed
-
- Backer LC, Fleming LE, Rowan A, Cheng YS, Benson J, Pierce R, Zaias J, Bean JA, Bossart JD, Johnson D, Quimbo R, Baden DG. Recreational exposure to aerosolized brevetoxins during Florida red tide events. Harmful Algae. 2003;2:19–28.
-
- Baden DG. Marine food-borne dinoflagellate toxins. Int. Rev. Cytol. 1983;82:99–150. - PubMed
-
- Baden DG, Fleming LE, Bean JA. Marine Toxins. In: deWolf FA, editor. Handbook of Clinical Neurology: Intoxications of the Nervous System Part H. Natural Toxins and Drugs. Elsevier Press; Amsterdam: 1995. pp. 141–175.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

