Altered biometal homeostasis is associated with CLN6 mRNA loss in mouse neuronal ceroid lipofuscinosis
- PMID: 23789114
- PMCID: PMC3683166
- DOI: 10.1242/bio.20134804
Altered biometal homeostasis is associated with CLN6 mRNA loss in mouse neuronal ceroid lipofuscinosis
Abstract
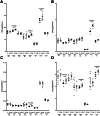
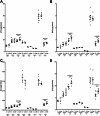
Neuronal ceroid lipofuscinoses, the most common fatal childhood neurodegenerative illnesses, share many features with more prevalent neurodegenerative diseases. Neuronal ceroid lipofuscinoses are caused by mutations in CLN genes. CLN6 encodes a transmembrane endoplasmic reticulum protein with no known function. We characterized the behavioural phenotype of spontaneous mutant mice modeling CLN6 disease, and demonstrate progressive motor and visual decline and reduced lifespan in these mice, consistent with symptoms observed in neuronal ceroid lipofuscinosis patients. Alterations to biometal homeostasis are known to play a critical role in pathology in Alzheimer's, Parkinson's, Huntington's and motor neuron diseases. We have previously shown accumulation of the biometals, zinc, copper, manganese and cobalt, in CLN6 Merino and South Hampshire sheep at the age of symptom onset. Here we determine the physiological and disease-associated expression of CLN6, demonstrating regional CLN6 transcript loss, and concurrent accumulation of the same biometals in the CNS and the heart of presymptomatic CLN6 mice. Furthermore, increased expression of the ER/Golgi-localized cation transporter protein, Zip7, was detected in cerebellar Purkinje cells and whole brain fractions. Purkinje cells not only control motor function, an early symptomatic change in the CLN6 mice, but also display prominent neuropathological changes in mouse models and patients with different forms of neuronal ceroid lipofuscinoses. Whole brain fractionation analysis revealed biometal accumulation in fractions expressing markers for ER, Golgi, endosomes and lysosomes of CLN6 brains. These data are consistent with a link between CLN6 expression and biometal homeostasis in CLN6 disease, and provide further support for altered cation transporter regulation as a key factor in neurodegeneration.
Keywords: Biometal homeostasis; CLN6; Metal transporter; Neurodegeneration; Neuronal ceroid lipofuscinoses.
Conflict of interest statement
Figures





Similar articles
-
Deregulation of subcellular biometal homeostasis through loss of the metal transporter, Zip7, in a childhood neurodegenerative disorder.Acta Neuropathol Commun. 2014 Feb 28;2:25. doi: 10.1186/2051-5960-2-25. Acta Neuropathol Commun. 2014. PMID: 24581221 Free PMC article.
-
Deregulation of biometal homeostasis: the missing link for neuronal ceroid lipofuscinoses?Metallomics. 2014 Apr;6(4):932-43. doi: 10.1039/c4mt00032c. Metallomics. 2014. PMID: 24804307
-
Disruption of the autophagy-lysosome pathway is involved in neuropathology of the nclf mouse model of neuronal ceroid lipofuscinosis.PLoS One. 2012;7(4):e35493. doi: 10.1371/journal.pone.0035493. Epub 2012 Apr 20. PLoS One. 2012. PMID: 22536393 Free PMC article.
-
The neuronal ceroid-lipofuscinoses.J Child Neurol. 1995 Nov;10(6):424-37. doi: 10.1177/088307389501000602. J Child Neurol. 1995. PMID: 8576551 Review.
-
Genetics of the neuronal ceroid lipofuscinoses (Batten disease).Biochim Biophys Acta. 2015 Oct;1852(10 Pt B):2237-41. doi: 10.1016/j.bbadis.2015.05.011. Epub 2015 May 27. Biochim Biophys Acta. 2015. PMID: 26026925 Free PMC article. Review.
Cited by
-
Oral treatment with Cu(II)(atsm) increases mutant SOD1 in vivo but protects motor neurons and improves the phenotype of a transgenic mouse model of amyotrophic lateral sclerosis.J Neurosci. 2014 Jun 4;34(23):8021-31. doi: 10.1523/JNEUROSCI.4196-13.2014. J Neurosci. 2014. PMID: 24899723 Free PMC article.
-
Unbiased Cell-based Screening in a Neuronal Cell Model of Batten Disease Highlights an Interaction between Ca2+ Homeostasis, Autophagy, and CLN3 Protein Function.J Biol Chem. 2015 Jun 5;290(23):14361-80. doi: 10.1074/jbc.M114.621706. Epub 2015 Apr 15. J Biol Chem. 2015. PMID: 25878248 Free PMC article.
-
Autophagy in the Neuronal Ceroid Lipofuscinoses (Batten Disease).Front Cell Dev Biol. 2022 Feb 16;10:812728. doi: 10.3389/fcell.2022.812728. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35252181 Free PMC article. Review.
-
Deregulation of subcellular biometal homeostasis through loss of the metal transporter, Zip7, in a childhood neurodegenerative disorder.Acta Neuropathol Commun. 2014 Feb 28;2:25. doi: 10.1186/2051-5960-2-25. Acta Neuropathol Commun. 2014. PMID: 24581221 Free PMC article.
-
Astrocytes and lysosomal storage diseases.Neuroscience. 2016 May 26;323:195-206. doi: 10.1016/j.neuroscience.2015.05.061. Epub 2015 May 30. Neuroscience. 2016. PMID: 26037807 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

