Efficacy of adalimumab across subgroups of patients with moderate-to-severe chronic plaque psoriasis of the hands and/or feet: post hoc analysis of REACH
- PMID: 23790018
- PMCID: PMC4229025
- DOI: 10.1111/jdv.12198
Efficacy of adalimumab across subgroups of patients with moderate-to-severe chronic plaque psoriasis of the hands and/or feet: post hoc analysis of REACH
Abstract
Background: The Randomized Controlled Evaluation of Adalimumab in Treatment of Chronic Plaque Psoriasis of the Hands and Feet (REACH) trial demonstrated that adalimumab was efficacious and well-tolerated for the treatment of hand and/or foot psoriasis through 28 weeks.
Objective: To evaluate the effects of patient baseline characteristics on efficacy of adalimumab treatment of hand and/or foot psoriasis.
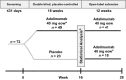
Methods: Patients with moderate-to-severe chronic plaque psoriasis of the hands and/or feet were randomized 2 : 1 to adalimumab or placebo during the 16 week, double-blind period of REACH. Primary endpoint was percentage of patients achieving Physician's Global Assessment of the hands and/or feet of clear/almost clear at week 16. Post hoc analyses evaluated effects of baseline patient characteristics on the primary endpoint. Patients with nail psoriasis at baseline were assessed for association of Nail Psoriasis Severity Index (NAPSI) 50 response with efficacy outcomes at week 16.
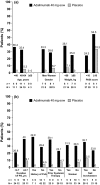
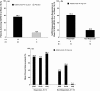
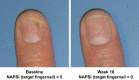
Results: Seventy-two patients (49 adalimumab : 23 placebo) were analysed. Greater percentages of adalimumab-treated patients achieved the primary endpoint vs. placebo across all subgroups. Among 31 patients with nail psoriasis, a greater percentage of adalimumab-treated patients achieved NAPSI 50 (56.5%) vs. placebo (12.5%) at week 16. In adalimumab-treated patients, greater percentages of NAPSI 50 Responders vs. Non-responders achieved the primary endpoint, and had greater improvements in erythema, scaling, induration and fissuring, Dermatology Life Quality Index, and pain scores.
Conclusions: Adalimumab was efficacious in treating chronic plaque psoriasis of the hands and/or feet over 16 weeks, regardless of baseline characteristics. Marked improvement in nail psoriasis among adalimumab-treated patients correlated with significant improvements in skin disease and patient-reported outcomes.
© 2013 AbbVie Inc. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of the European Academy of Dermatology and Venereology.
Figures
References
-
- Kumar B, Saraswat A, Kaur I. Palmoplantar lesions in psoriasis: a study of 3065 patients. Acta Derm Venereol. 2002;82:192–195. - PubMed
-
- Jiaravuthisan MM, Sasseville D, Vender RB, et al. Psoriasis of the nail: anatomy, pathology, clinical presentation, and a review of the literature on therapy. J Am Acad Dermatol. 2007;57:1–27. - PubMed
-
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826–850. - PubMed
-
- Farley E, Masrour S, McKey J, Menter A. Palmoplantar psoriasis: a phenotypical and clinical review with introduction of a new quality-of-life assessment tool. J Am Acad Dermatol. 2009;60:1024–1031. - PubMed
-
- Greaves MW, Weinstein GD. Treatment of psoriasis. N Engl J Med. 1995;332:581–588. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

