Repeated ketamine exposure induces an enduring resilient phenotype in adolescent and adult rats
- PMID: 23790225
- PMCID: PMC3785550
- DOI: 10.1016/j.biopsych.2013.04.027
Repeated ketamine exposure induces an enduring resilient phenotype in adolescent and adult rats
Abstract
Background: Major depressive disorder afflicts up to 10% of adolescents. However, nearly 50% of those afflicted are considered nonresponsive to available treatments. Ketamine, a noncompetitive N-methyl-D-aspartate receptor antagonist has shown potential as a rapid-acting and long-lasting treatment for major depressive disorder in adults. Thus, the effectiveness and functional consequences of ketamine exposure during adolescence were explored.
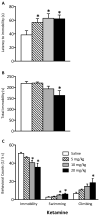
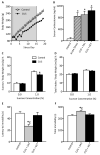
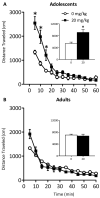
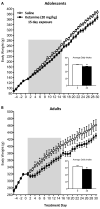
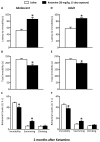
Methods: Adolescent male rats (postnatal day [PD] 35) received two ketamine (0, 5, 10, or 20 mg/kg) injections, 4 hours apart, after exposure to day 1 of the forced swim test (FST). The next day, rats were reexposed to the FST to assess ketamine-induced antidepressant-like responses. Separate groups were exposed to chronic unpredictable stress to confirm findings from the FST. After these initial experiments, adolescent naive rats were exposed to either 1 or 15 consecutive days (PD35-49) of ketamine (20 mg/kg) twice daily. Ketamine's influence on behavioral reactivity to rewarding (i.e., sucrose preference) and aversive (i.e., elevated plus-maze, FST) circumstances was then assessed 2 months after treatment. To control for age-dependent effects, adult rats (PD75-89) were exposed to identical experimental conditions.
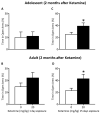
Results: Ketamine (20 mg/kg) reversed the chronic unpredictable stress-induced depression-like behaviors in the FST. Repeated ketamine exposure resulted in anxiolytic- and antidepressant-like responses 2 months after drug exposure. None of the ketamine doses used were capable of inducing drug-seeking behaviors as measured by place preference conditioning.
Conclusions: Repeated ketamine exposure induces enduring resilient-like responses regardless of age of exposure. These findings point to ketamine, and its repeated exposure, as a potentially useful antidepressant during adolescence.
Keywords: Adolescence; anxiety; depression; ketamine; rats; resilience; stress.
© 2013 Society of Biological Psychiatry.
Figures
Comment in
-
Scopolamine and ketamine: evidence of convergence?Biol Psychiatry. 2013 Nov 15;74(10):712-3. doi: 10.1016/j.biopsych.2013.08.011. Biol Psychiatry. 2013. PMID: 24144324 No abstract available.
References
-
- Hyman S, Chisholm D, Kessler R, Patel V, Whiteford H. Mental Disorders. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, et al., editors. Disease Control Priorities in Developing Countries. 2nd ed. Washington (DC): 2006. - PubMed
-
- Murray CJ, Lopez AD. Evidence-based health policy--lessons from the Global Burden of Disease Study. Science. 1996;274:740–743. - PubMed
-
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. - PubMed
-
- Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

