γ-Secretase inhibitors and modulators
- PMID: 23791707
- PMCID: PMC3857966
- DOI: 10.1016/j.bbamem.2013.06.005
γ-Secretase inhibitors and modulators
Abstract
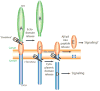
γ-Secretase is a fascinating, multi-subunit, intramembrane cleaving protease that is now being considered as a therapeutic target for a number of diseases. Potent, orally bioavailable γ-secretase inhibitors (GSIs) have been developed and tested in humans with Alzheimer's disease (AD) and cancer. Preclinical studies also suggest the therapeutic potential for GSIs in other disease conditions. However, due to inherent mechanism based-toxicity of non-selective inhibition of γ-secretase, clinical development of GSIs will require empirical testing with careful evaluation of benefit versus risk. In addition to GSIs, compounds referred to as γ-secretase modulators (GSMs) remain in development as AD therapeutics. GSMs do not inhibit γ-secretase, but modulate γ-secretase processivity and thereby shift the profile of the secreted amyloid β peptides (Aβ) peptides produced. Although GSMs are thought to have an inherently safe mechanism of action, their effects on substrates other than the amyloid β protein precursor (APP) have not been extensively investigated. Herein, we will review the current state of development of GSIs and GSMs and explore pertinent biological and pharmacological questions pertaining to the use of these agents for select indications. This article is part of a Special Issue entitled: Intramembrane Proteases.
Keywords: Alzheimer's disease; Cancer; γ-Secretase inhibitor; γ-Secretase modulator.
Copyright © 2013. Published by Elsevier B.V.
Figures
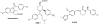
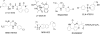
References
-
- Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, Takahashi Y, Thinakaran G, Iwatsubo T. The role of presenilin cofactors in the gamma-secretase complex. Nature. 2003;422:438–441. - PubMed
-
- De Strooper B. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma- Secretase complex. Neuron. 2003;38:9–12. - PubMed
-
- Wolfe MS, Kopan R. Intramembrane proteolysis: theme and variations. Science. 2004;305:1119–1123. - PubMed
-
- Haapasalo A, Kovacs DM. The Many Substrates of Presenilin/gamma-Secretase. J Alzheimers Dis. 2011
-
- Shoji M, Golde TE, Ghiso J, Cheung TT, Estus S, Shaffer LM, Cai XD, McKay DM, Tintner R, Frangione B, et al. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992;258:126–129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

