The immune system and inflammation in breast cancer
- PMID: 23791814
- PMCID: PMC4919022
- DOI: 10.1016/j.mce.2013.06.003
The immune system and inflammation in breast cancer
Abstract
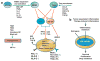
During different stages of tumor development the immune system can either identify and destroy tumors, or promote their growth. Therapies targeting the immune system have emerged as a promising treatment modality for breast cancer, and immunotherapeutic strategies are being examined in preclinical and clinical models. However, our understanding of the complex interplay between cells of the immune system and breast cancer cells is incomplete. In this article, we review recent findings showing how the immune system plays dual host-protective and tumor-promoting roles in breast cancer initiation and progression. We then discuss estrogen receptor α (ERα)-dependent and ERα-independent mechanisms that shield breast cancers from immunosurveillance and enable breast cancer cells to evade immune cell induced apoptosis and produce an immunosuppressive tumor microenvironment. Finally, we discuss protumorigenic inflammation that is induced during tumor progression and therapy, and how inflammation promotes more aggressive phenotypes in ERα positive breast cancers.
Keywords: Breast cancer; ER-alpha; Immunity; Immunosurveillance; Inflammation.
Copyright © 2013. Published by Elsevier Ireland Ltd.
Figures



References
-
- Bargou RC, Wagener C, Bommert K, Mapara MY, Daniel PT, Arnold W, Dietel M, Guski H, Feller A, Royer HD, Dorken B. Overexpression of the death-promoting gene bax-alpha which is downregulated in breast cancer restores sensitivity to different apoptotic stimuli and reduces tumor growth in SCID mice. J Clin Invest. 1996;97:2651–2659. - PMC - PubMed
-
- Basu S, Nachat-Kappes R, Caldefie-Chezet F, Vasson MP. Eicosanoids and adipokines in breast cancer: from molecular mechanisms to clinical considerations. Antioxid Redox Signal 2012 - PubMed
-
- Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–5380. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

