Exposure to chemical cocktails before or after conception--- the effect of timing on ovarian development
- PMID: 23791816
- PMCID: PMC3731555
- DOI: 10.1016/j.mce.2013.06.016
Exposure to chemical cocktails before or after conception--- the effect of timing on ovarian development
Abstract
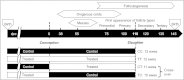
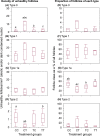
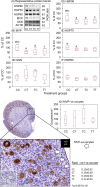
Exposure of female fetuses to environmental chemicals (ECs) during pregnancy results in a disturbed ovarian adult phenotype. We investigated the influence of pre- and/or post-conception exposure to low-level mixtures of ECs on the structure and function of the fetal ovine ovary. We examined ovarian morphology, expression of oocyte and granulosa cell-specific genes and proteome. Female fetuses were collected at day 110 of gestation, from dams exposed continuously until, and after mating, by grazing in pastures treated with sewage sludge as a fertiliser (TT) or in control fields treated with inorganic fertiliser (CC). In addition, in a cross-over design, fetal ovaries were collected from dams maintained on sludge pastures up to the time of mating but then transferred to control pastures (TC) and, reciprocally, those transferred from control to treated pastures at mating (CT). On examination, the proportion of type 1a follicles (activating primordial follicles) was significantly lower in animals from the CT groups compared with CC and TT groups (P<0.05). Of the 23 ovarian gene transcripts studied, 14 were altered in the ovaries of exposed fetuses (CT, TC, and TT) relative to controls, with the largest number of changes observed in cross-exposure pattern groups (CT or TC). Continuous EC exposure (TT) produced fewer transcript alterations and only two genes (INHBA and GSN) presented differential profiles between CC and TT. Fetal ovarian proteome analysis (2-DE gels) showed, across all exposure groups, 86 differentially expressed protein spots compared to controls. Animals in the CT group exhibited the highest number (53) while TC and TT presented the same number of affected protein spots (42). Fetal ovarian proteins with altered expression included MVP (major vault protein) and several members of the heat-shock family (HSPA4L, HSP90AA1 and HSF1). The present findings indicate that continuous maternal EC exposure before and during gestation, are less deleterious for fetal ovarian development than a change in maternal EC exposure between pre and post-conception. The pathways by which the ovary responds to this chemical stress were common in TT, CT, TC exposed foetuses. In addition to the period of pregnancy, the pre-conception period appears also as crucial for conditioning long-term effects of EC exposure on ovarian development and primordial follicle reserve and hence future fertility.
Keywords: Anti-ACTB; DEHP; Development; ECs; EDCs; Environmental chemicals; FSH; In utero exposure; LH; Mixtures; Ovary; WB; Western blot; anti-β actin; diethylhexylphthalate; endocrine disrupting chemicals; environmental chemicals; follicle stimulating hormone; luteinising hormone.
Copyright © 2013 The Authors. Published by Elsevier Ireland Ltd.. All rights reserved.
Figures




References
-
- Adhikari D., Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr. Rev. 2009;30:438–464. - PubMed
-
- Alcorn J., McNamara P.J. Ontogeny of hepatic and renal systemic clearance pathways in infants: Part II. Clin. Pharmacokinet. 2002;41:1077–1094. - PubMed
-
- Alcorn J., McNamara P.J. Pharmacokinetics in the newborn. Adv. Drug Deliv. Rev. 2003;55:667–686. - PubMed
-
- Bellingham M., Fowler P.A., Amezaga M.R., Rhind S.M., Cotinot C., Mandon-Pepin B., Sharpe R.M., Evans N.P. Exposure to a complex cocktail of environmental endocrine-disrupting compounds disturbs the kisspeptin/GPR54 system in ovine hypothalamus and pituitary gland. Environ. Health Perspect. 2009;117:1556–1562. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

