Cell-type-specific profiling of gene expression and chromatin binding without cell isolation: assaying RNA Pol II occupancy in neural stem cells
- PMID: 23792147
- PMCID: PMC3714590
- DOI: 10.1016/j.devcel.2013.05.020
Cell-type-specific profiling of gene expression and chromatin binding without cell isolation: assaying RNA Pol II occupancy in neural stem cells
Abstract
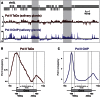
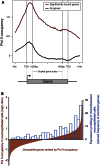
Cell-type-specific transcriptional profiling often requires the isolation of specific cell types from complex tissues. We have developed "TaDa," a technique that enables cell-specific profiling without cell isolation. TaDa permits genome-wide profiling of DNA- or chromatin-binding proteins without cell sorting, fixation, or affinity purification. The method is simple, sensitive, highly reproducible, and transferable to any model system. We show that TaDa can be used to identify transcribed genes in a cell-type-specific manner with considerable temporal precision, enabling the identification of differential gene expression between neuroblasts and the neuroepithelial cells from which they derive. We profile the genome-wide binding of RNA polymerase II in these adjacent, clonally related stem cells within intact Drosophila brains. Our data reveal expression of specific metabolic genes in neuroepithelial cells, but not in neuroblasts, and highlight gene regulatory networks that may pattern neural stem cell fates.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Almeida M.S., Bray S.J. Regulation of post-embryonic neuroblasts by Drosophila Grainyhead. Mech. Dev. 2005;122:1282–1293. - PubMed
-
- Bardin A.J., Schweisguth F. Bearded family members inhibit Neuralized-mediated endocytosis and signaling activity of Delta in Drosophila. Dev. Cell. 2006;10:245–255. - PubMed
-
- Bonn S., Zinzen R.P., Girardot C., Gustafson E.H., Perez-Gonzalez A., Delhomme N., Ghavi-Helm Y., Wilczyński B., Riddell A., Furlong E.E. Tissue-specific analysis of chromatin state identifies temporal signatures of enhancer activity during embryonic development. Nat. Genet. 2012;44:148–156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

