Transport dynamics in a glutamate transporter homologue
- PMID: 23792560
- PMCID: PMC3829612
- DOI: 10.1038/nature12265
Transport dynamics in a glutamate transporter homologue
Abstract
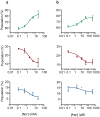
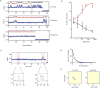
Glutamate transporters are integral membrane proteins that catalyse neurotransmitter uptake from the synaptic cleft into the cytoplasm of glial cells and neurons. Their mechanism of action involves transitions between extracellular (outward)-facing and intracellular (inward)-facing conformations, whereby substrate binding sites become accessible to either side of the membrane. This process has been proposed to entail transmembrane movements of three discrete transport domains within a trimeric scaffold. Using single-molecule fluorescence resonance energy transfer (smFRET) imaging, we have directly observed large-scale transport domain movements in a bacterial homologue of glutamate transporters. We find that individual transport domains alternate between periods of quiescence and periods of rapid transitions, reminiscent of bursting patterns first recorded in single ion channels using patch-clamp methods. We propose that the switch to the dynamic mode in glutamate transporters is due to separation of the transport domain from the trimeric scaffold, which precedes domain movements across the bilayer. This spontaneous dislodging of the substrate-loaded transport domain is approximately 100-fold slower than subsequent transmembrane movements and may be rate determining in the transport cycle.
Figures


References
-
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1. - PubMed
-
- Weiss S. Fluorescence spectroscopy of single biomolecules. Science. 1999;283(5408):1676. - PubMed
-
- Sakmann B, Patlak J, Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980;286(5768):71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

