Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling
- PMID: 23792629
- PMCID: PMC3814166
- DOI: 10.1038/nbt.2614
Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling
Abstract
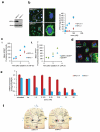
Despite efforts to understand the interactions between nanoparticles and cells, the cellular processes that determine the efficiency of intracellular drug delivery remain unclear. Here we examine cellular uptake of short interfering RNA (siRNA) delivered in lipid nanoparticles (LNPs) using cellular trafficking probes in combination with automated high-throughput confocal microscopy. We also employed defined perturbations of cellular pathways paired with systems biology approaches to uncover protein-protein and protein-small molecule interactions. We show that multiple cell signaling effectors are required for initial cellular entry of LNPs through macropinocytosis, including proton pumps, mTOR and cathepsins. siRNA delivery is substantially reduced as ≅70% of the internalized siRNA undergoes exocytosis through egress of LNPs from late endosomes/lysosomes. Niemann-Pick type C1 (NPC1) is shown to be an important regulator of the major recycling pathways of LNP-delivered siRNAs. NPC1-deficient cells show enhanced cellular retention of LNPs inside late endosomes and lysosomes, and increased gene silencing of the target gene. Our data suggest that siRNA delivery efficiency might be improved by designing delivery vehicles that can escape the recycling pathways.
Figures

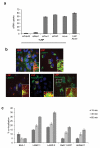

 , siRNA complex-
, siRNA complex- )
)Comment in
-
A window onto siRNA delivery.Nat Biotechnol. 2013 Jul;31(7):611-2. doi: 10.1038/nbt.2634. Nat Biotechnol. 2013. PMID: 23839146 No abstract available.
-
Research highlights: Highlights from the last year in nanomedicine.Nanomedicine (Lond). 2014 Jan;9(1):17-20. doi: 10.2217/nnm.13.188. Nanomedicine (Lond). 2014. PMID: 24354812 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

